Types of OxidationReduction Reactions Combination Reaction Decomposition reaction
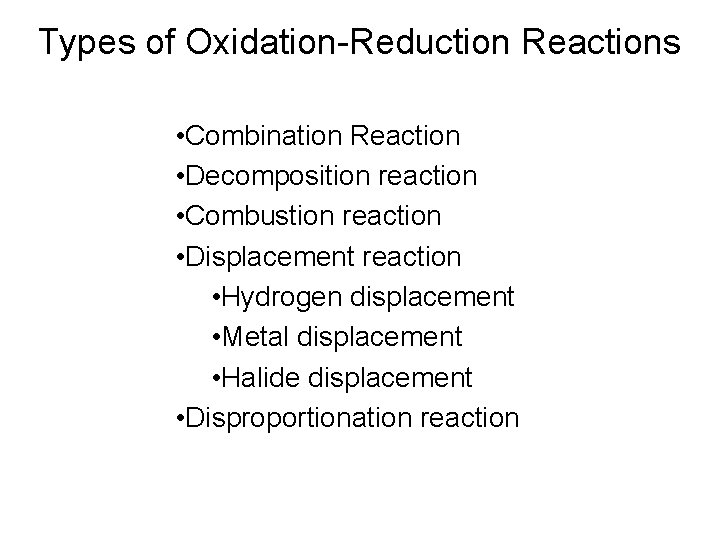
Types of Oxidation-Reduction Reactions • Combination Reaction • Decomposition reaction • Combustion reaction • Displacement reaction • Hydrogen displacement • Metal displacement • Halide displacement • Disproportionation reaction
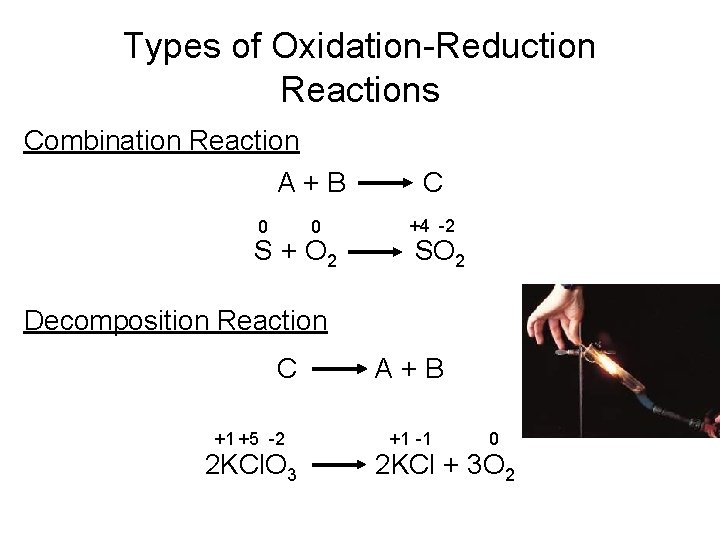
Types of Oxidation-Reduction Reactions Combination Reaction A+B C 0 +4 -2 0 S + O 2 SO 2 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2
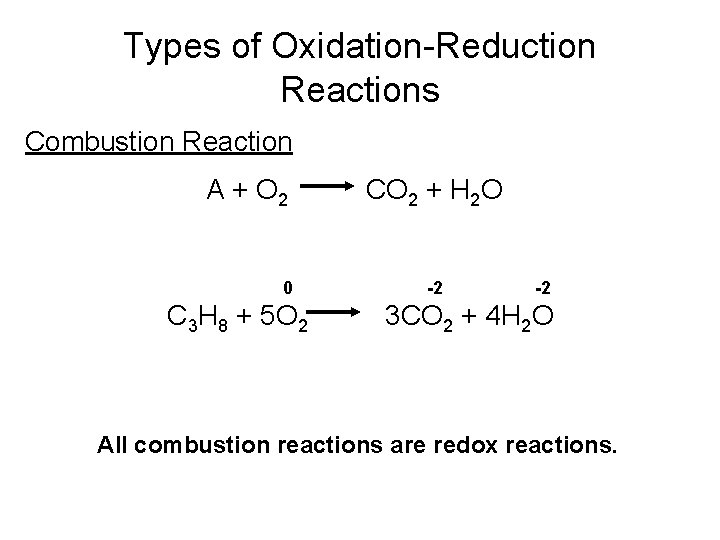
Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 0 C 3 H 8 + 5 O 2 CO 2 + H 2 O -2 -2 3 CO 2 + 4 H 2 O All combustion reactions are redox reactions.
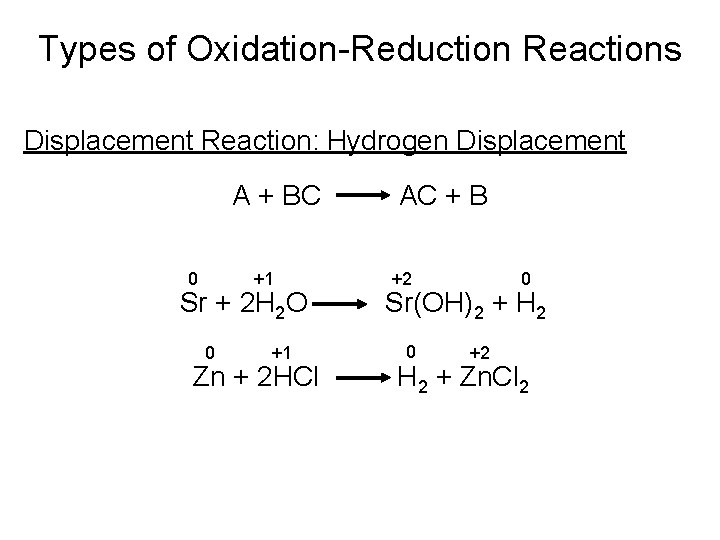
Types of Oxidation-Reduction Reactions Displacement Reaction: Hydrogen Displacement A + BC +1 0 Sr + 2 H 2 O 0 +1 Zn + 2 HCl AC + B +2 0 Sr(OH)2 + H 2 0 +2 H 2 + Zn. Cl 2
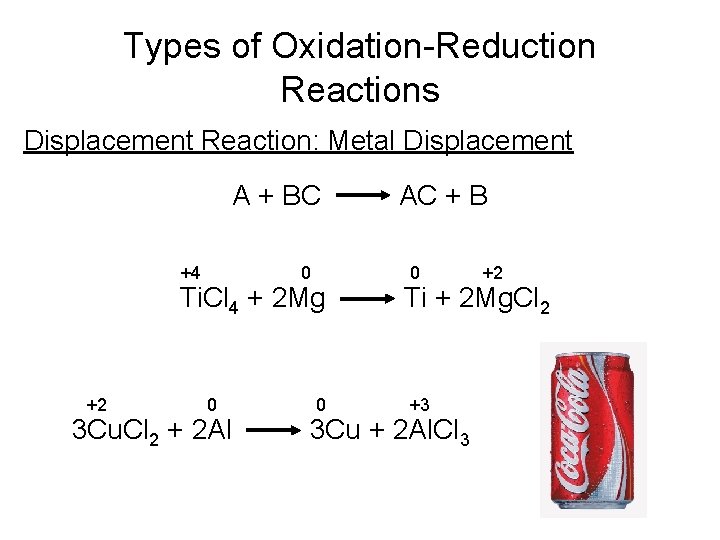
Types of Oxidation-Reduction Reactions Displacement Reaction: Metal Displacement A + BC +4 0 Ti. Cl 4 + 2 Mg +2 0 3 Cu. Cl 2 + 2 Al 0 AC + B 0 +2 Ti + 2 Mg. Cl 2 +3 3 Cu + 2 Al. Cl 3
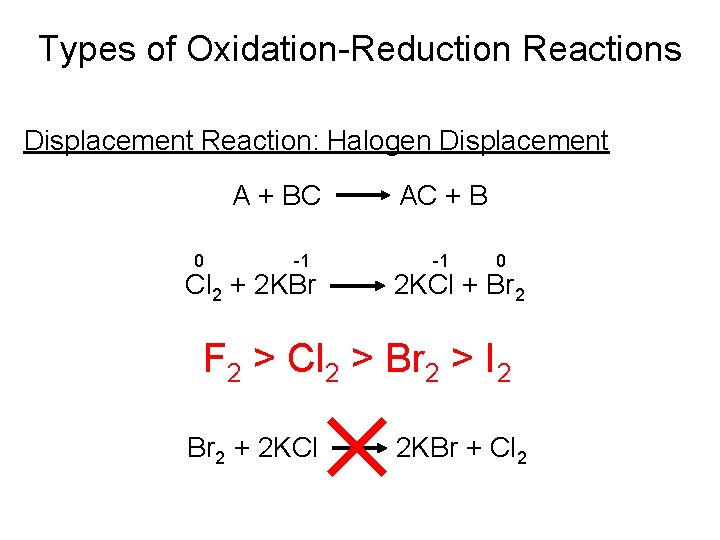
Types of Oxidation-Reduction Reactions Displacement Reaction: Halogen Displacement A + BC 0 -1 Cl 2 + 2 KBr AC + B -1 0 2 KCl + Br 2 F 2 > Cl 2 > Br 2 > I 2 Br 2 + 2 KCl 2 KBr + Cl 2
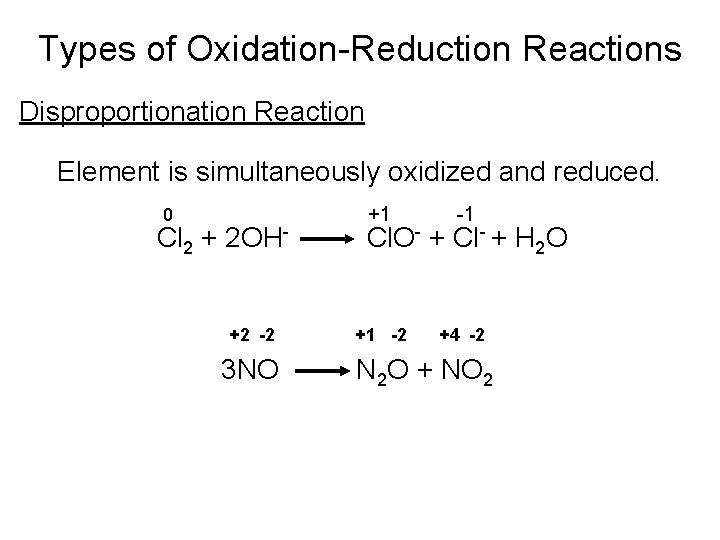
Types of Oxidation-Reduction Reactions Disproportionation Reaction Element is simultaneously oxidized and reduced. 0 Cl 2 + 2 OH+2 -2 3 NO +1 -1 +1 -2 +4 -2 Cl. O- + Cl- + H 2 O N 2 O + NO 2
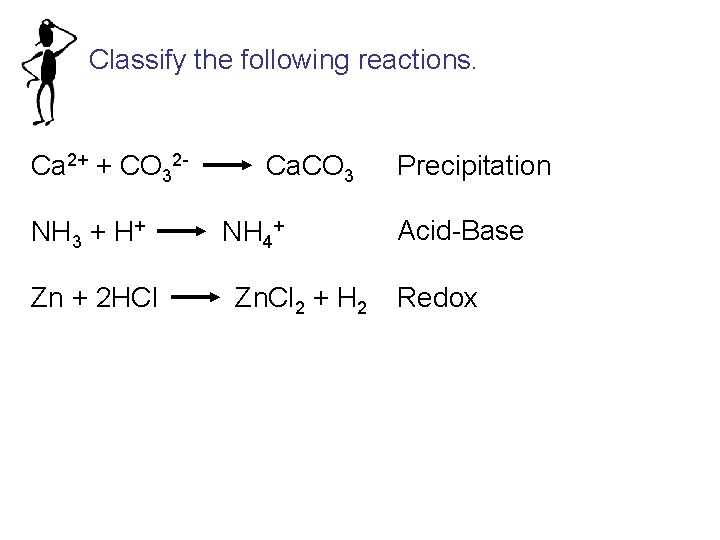
Classify the following reactions. Ca 2+ + CO 32 NH 3 + H+ Zn + 2 HCl Ca. CO 3 NH 4+ Zn. Cl 2 + H 2 Precipitation Acid-Base Redox

Household electron transfer: bleach, oxiclean, etc http: //tooth-whitening-site. info/images/teeth 3. jpg http: //oxygenbleach. homestead. com
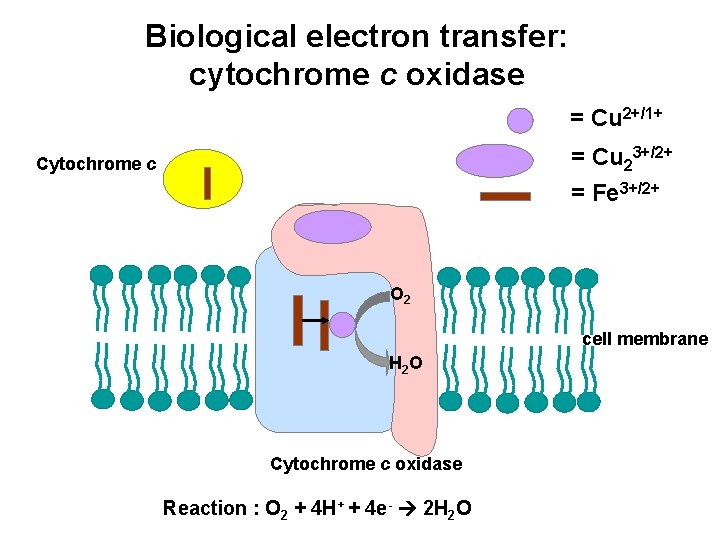
Biological electron transfer: cytochrome c oxidase = Cu 2+/1+ = Cu 23+/2+ = Fe 3+/2+ Cytochrome c O 2 cell membrane H 2 O Cytochrome c oxidase Reaction : O 2 + 4 H+ + 4 e- 2 H 2 O
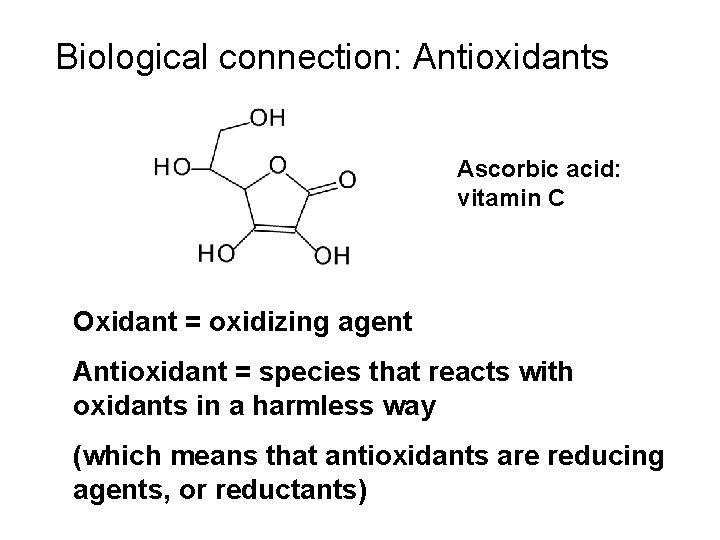
Biological connection: Antioxidants Ascorbic acid: vitamin C Oxidant = oxidizing agent Antioxidant = species that reacts with oxidants in a harmless way (which means that antioxidants are reducing agents, or reductants)

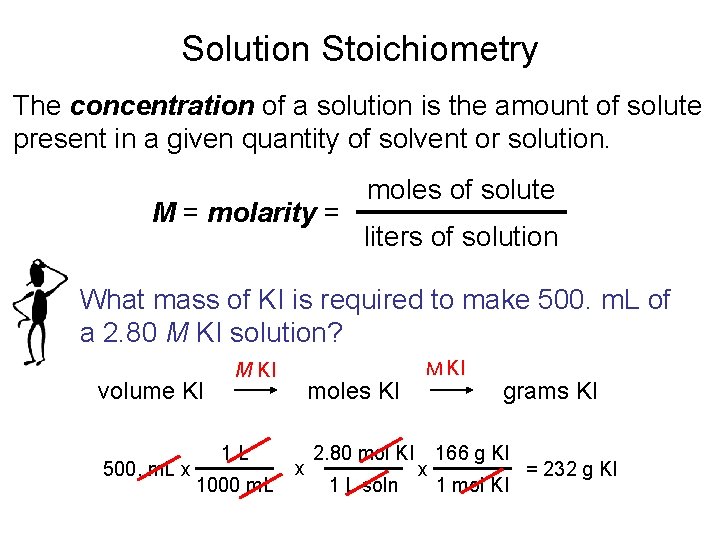
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? volume KI 500. m. L x M KI 1 L 1000 m. L moles KI x 2. 80 mol KI 1 L soln M KI x grams KI 166 g KI 1 mol KI = 232 g KI
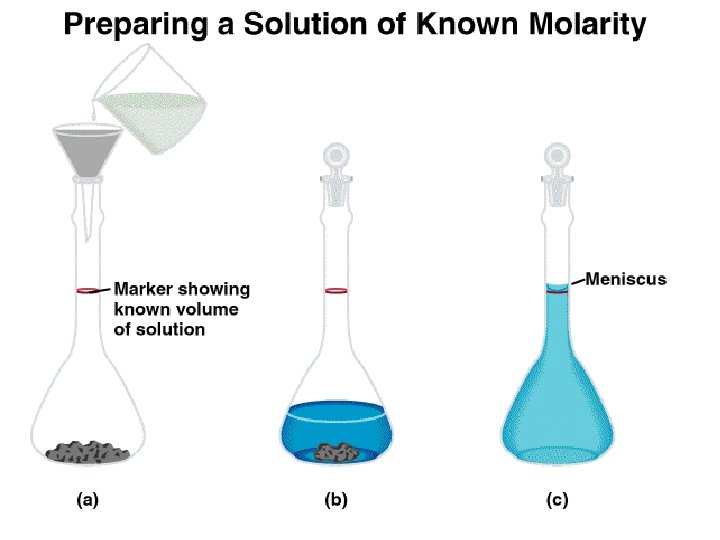
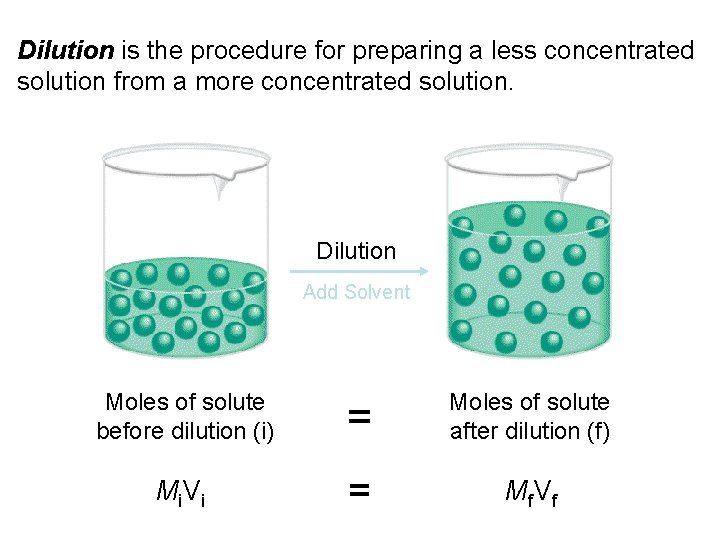
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f
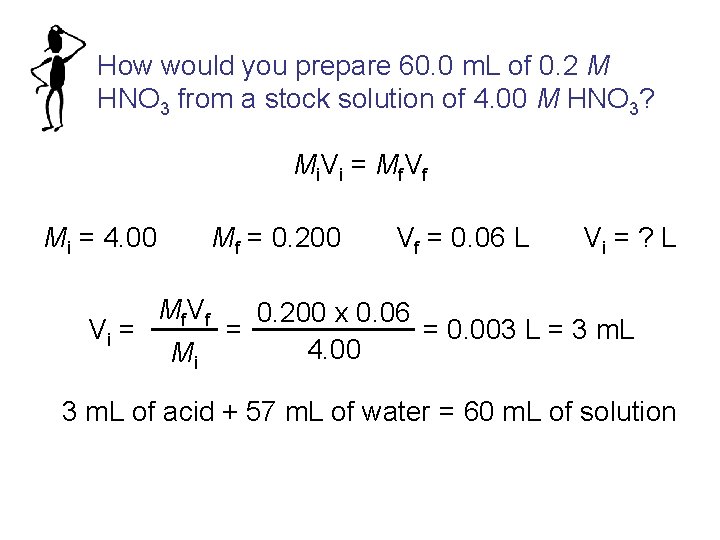
How would you prepare 60. 0 m. L of 0. 2 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi V i = Mf V f Mi = 4. 00 Vi = Mf = 0. 200 Mf V f Mi Vf = 0. 06 L Vi = ? L 0. 200 x 0. 06 = 0. 003 L = 3 m. L = 4. 00 3 m. L of acid + 57 m. L of water = 60 m. L of solution
- Slides: 16