Types of Ophthalmic Dosage Forms Liquid Dosage Form










- Slides: 16

Types of Ophthalmic Dosage Forms Liquid Dosage Form Semisolid Dosage Form Solution Ointment Suspension Emulsion Powders for reconstitution Solid Dosage Form Insert

Semi solid Dosage Forms Ophthalmic Ointment Interfere with vision unless use is limited to bed time instillation. l Often used as adjunctive night time therapy, with eye drops administered during the day. l l Longer contact time and greater total drug bioavailability, with slower onset & much more time to reach peak absorption. Vehicle is usually a mixture of mineral oil & white petrolatum. Mineral oil is added to: a)reduce the melting point. b)Modify the consistency. l

Petrolatum-based ointments are: a)Bland. b)Inert nature which make them suitable vehicle for moisture -sensitive drugs.

l Ointments used as vehicles for antibiotics, sulfonamides, antiviral, antifungal, & antiinflammatory corticosteroids. l Anesthesiologists may prescribe the ointment vehicle for the surgical patient undergoing general anesthesia to prevent severe and painful dry eye conditions. l The anhydrous petrolatum base may be made more miscible with water through the use of an anhydrous liquid lanolin derivative , then aqueous drug solution can be incorporated.

How to use ophthalmic ointment? 1. 2. 3. 4. 5. 6. 7. Wash hands. Remove cap from the tube. With one hand, gently pull the lower eye lid down. While looking up, squeeze a small amount of ointment (about ¼ to ½ inch) inside lower lid. Be careful not to touch tip of tube to eye, eyelid, fingers, etc. Close eye gently and roll eyeball in all directions while eye is closed. Temporary blurring may occur. The closed eye lid may be rubbed very gently by a finger to distribute the drug throughout the fornix. Replace cap on tube.

Types of Ophthalmic Dosage Forms Liquid Dosage Form Solution Suspension Emulsion Powders for reconstitution Semisolid Dosage Form Ointment Solid Dosage Form Insert

Solid Dosage Forms Ocular Inserts

Non erodible Ocular Inserts l In 1975, the first controlled release topical dosage form was marketed in the United States by the Alza Corporation which was pilocarpine ocusert. l Pilocarpine ocusert is an elliptical- shaped membrane which is soft & flexible designed to be placed in the cul-de-sac between the sclera & the eye lid.

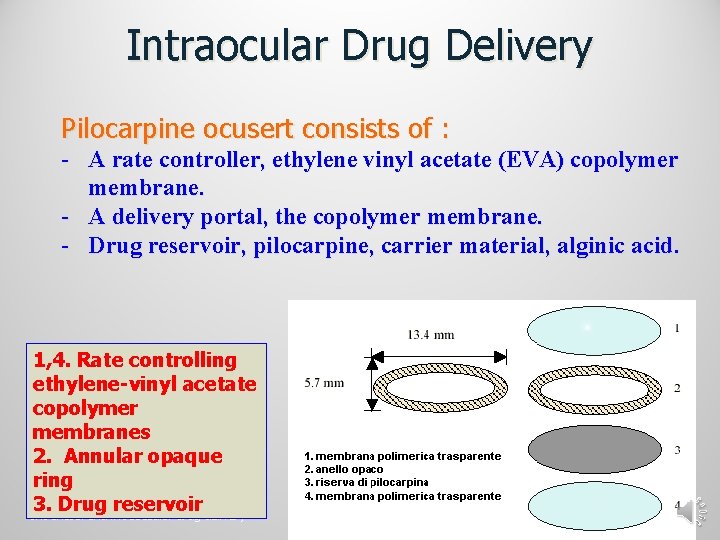
Intraocular Drug Delivery Pilocarpine ocusert consists of : - A rate controller, ethylene vinyl acetate (EVA) copolymer membrane. - A delivery portal, the copolymer membrane. - Drug reservoir, pilocarpine, carrier material, alginic acid. 1, 4. Rate controlling ethylene-vinyl acetate copolymer membranes 2. Annular opaque ring 3. Drug reservoir Intranasal and intraoccular drug delivery 03/10/2009

• Design 0 f ocusert: üThe ocusert is sterile, oval in shape & flexible, üIt is made of a central core or reservoir which contains the drug embedded in an alginic acid gel-like base, üthe drug reservoir is surrounded by a rate-limiting or controlling membrane made of ethylene vinyl acetate copolymer which gives a steady and controlled drug release for a certain period of time. üThe device is bordered by a white annular ring consisting of ethylene vinyl acetate co-polymer impregnated with titanium dioxide (a powdered pigment) that darkens the borders of the ocusert. The border makes the ocusert easier for the patient to visualize.

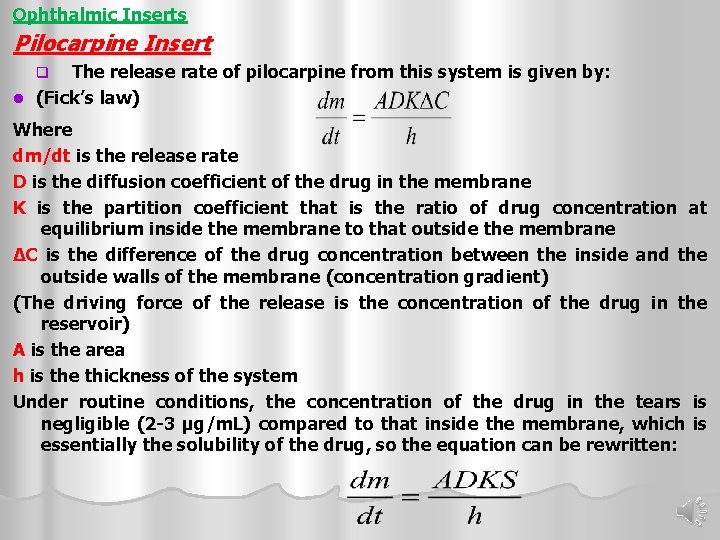
Ophthalmic Inserts Pilocarpine Insert The release rate of pilocarpine from this system is given by: l (Fick’s law) q Where dm/dt is the release rate D is the diffusion coefficient of the drug in the membrane K is the partition coefficient that is the ratio of drug concentration at equilibrium inside the membrane to that outside the membrane ΔC is the difference of the drug concentration between the inside and the outside walls of the membrane (concentration gradient) (The driving force of the release is the concentration of the drug in the reservoir) A is the area h is the thickness of the system Under routine conditions, the concentration of the drug in the tears is negligible (2 -3 µg/m. L) compared to that inside the membrane, which is essentially the solubility of the drug, so the equation can be rewritten:

Pilocarpine ocusert • Used to treat Glaucoma because it reduces intraocular pressure • It is designed to release pilocarpine at a controlled required rate (for example, 20 micrograms hour or 40 microgram or 80 microgram per hour), for a certain period of time (like 7 days), after 7 days the ocusert is removed & replaced by a new one, so it’s more convenient than pilocarpine eye drops which is used 4 times a day normally. Advantages over drop therapy for the glaucoma patient: Exposes the patient to only one-fourth to one-eighth the amount of pilocarpine compared to drop therapy. (reduced local side effects & toxicity. ) It provides a continuous around – the clock- control of IOP. Patient convenience & improved compliance. (administered only once per week. )

Erodible Ocular Inserts Why ? 1. They do not have to be removed at the end of therapy 2. Increase retention time 3. Increase penetration of the drug 4. Prolonged effect Ex. Lacrisert l

Lacrisert used to treat dry eyes, keratitis, and decreased corneal sensitivity It contain 5 mg of HPMC in a rodshaped of about 1. 27 mm diameter and 3. 5 mm long, no preservative since it is anhydrous. It imbibes water from the tears and forms a gel-like mass after several hours, which gradually erodes as the polymer dissolves, it thickens the tear film and provides increased lubrication. it used once or twice daily

Soft Contact Lens Soft hydrophilic contact lens of the hydroxyl ethyl methacrylate (HEMA) polymer to prolong delivery of pilocarpine. l Lens is presoaked in a sterile unpreserved solution of the drug. l Placed in the eye over the cornea for a period of time, usually few minutes to several hours to increase the amount of drug absorbed or to prolong the duration of effect. (reduce the frequency of drug instillation & give diurnal control to the treatment of glaucoma)

• Disadvantages: Uncontrolled nature to the release of drug from it. Potential for increased risks of contamination since unpreserved drug solutions must be used & the patient must disinfect the lens himself. l Example of contact lenses l The Bionite lens which is made from hydrophilic polymer (2 -hydroxy ethyl methacrylate ) has been shown to produce a greater penetration of fluorescein.