Types of Mixtures The saga continues Mixtures n

























- Slides: 29

Types of Mixtures The saga continues….

Mixtures n n n Combination of more than one pure substance but not bonded chemically Can be separated physically or chemically! Salads, Kool-Aid, Pizza.

Types of Mixtures: Homogeneous n “Homogeneous” means: appears the same throughout. n n Kool Aid contains water, sugar & coloring, but is not a pure substance because each of the ingredients can vary each time it is made. It is a homogeneous mixture because it is the same throughout and can be separated! We say they are “miscible” (they dissolve completely).

Types of Mixtures: Heterogeneous n “Heterogeneous” Mixture: Mixtures in which differences can be seen easily…oil & water, sand & water, flour & water.

Mixtures also come in Different Forms n There are THREE Forms of mixtures: n. Solutions n. Suspensions n. Colloids

Solutions n A solution is a mixture that appears to be a single substance, but it is actually composed of 2 or more substances that are distributed evenly amongst each other. n SOLUTIONS ARE HOMOGENEOUS carbon dioxide is dissolved in water sugar & Kool-Aid powder are dissolved in water salt is dissolved in water

Suspensions n A suspension is a mixture in which particles of a material are dispersed throughout a liquid or gas, but are large enough that they settle out. n SUSPENSIONS ARE HETEROGENEOUS muddy water Snow globe Italian dressing

Colloids n A colloid is a mixture in which the particles are dispersed throughout but are not heavy enough to settle out. n COLLOIDS ARE HOMOGENEOUS Colloids have properties of both solutions & suspension s. mayonnaise milk

Separating Mixtures n n Substances in a mixture are physically combined, so processes bases on differences in physical properties are used to separate component Numerous techniques have been developed to separate mixtures to study components

• Filtration • Sieving • Dissolving • Centrifuging • Evaporating • Distillation • Magnet 10

Filtration n Used to separate heterogeneous mixtures composed of solids and liquids Uses a porous barrier to separate the solid from the liquid Liquid passes through leaving the solid in the filter paper

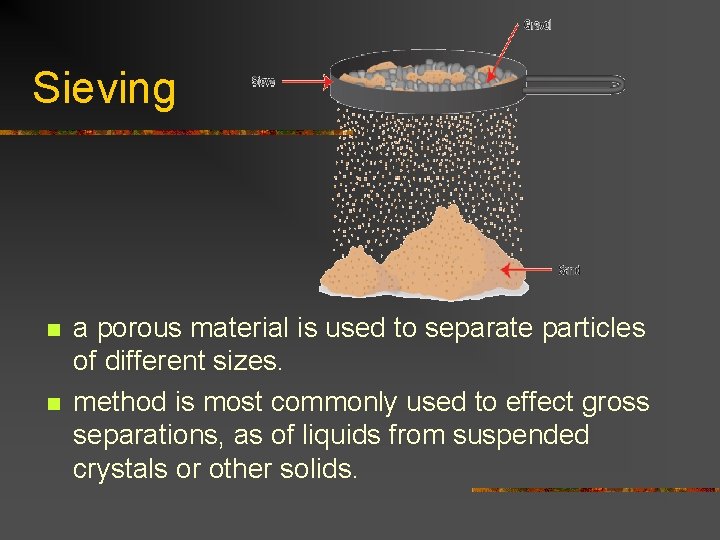
Sieving n n a porous material is used to separate particles of different sizes. method is most commonly used to effect gross separations, as of liquids from suspended crystals or other solids.

Dissolving n Used to separate two solids when one is soluble in water and the other is not n Put the solids in a solvent(water) and then use a filter to separate the insoluable solid

Evaporation can be used to separate a solute from the solvent in a solution

Distillation n n Used to separate homogeneous mixtures Based on differences in boiling points of substances involved

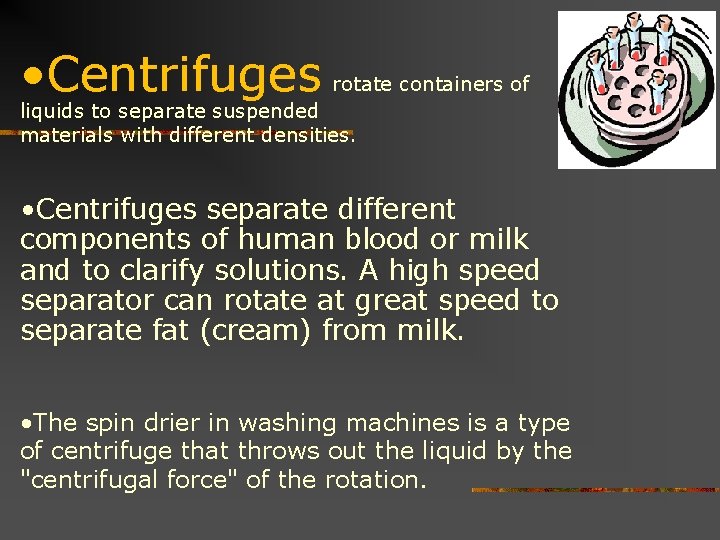
• Centrifuges rotate containers of liquids to separate suspended materials with different densities. • Centrifuges separate different components of human blood or milk and to clarify solutions. A high speed separator can rotate at great speed to separate fat (cream) from milk. • The spin drier in washing machines is a type of centrifuge that throws out the liquid by the "centrifugal force" of the rotation. 16

A magnet n Can be used to separate a magnetic substance from a non-magnetic substance

What is it? Cheerios

Homogeneous

What is it? Trail Mix

Heterogeneous

What is it? Kool - Aid

Homogeneous

What is it? Italian Salad Dressing

Heterogeneous

Try these………. . Raisin Bran

Orange Juice

Air

Cake Batter