Types of Mixtures Mixtures A combination of two

Types of Mixtures

Mixtures • A combination of two or more substances that do not combine chemically, but remain the same individual substances; can be separated by physical means • Two types: – Heterogeneous – Homogeneous Based on the prefixes “hetero” and “homo, ” what do you think are characteristics of these two types of mixtures?

Heterogeneous Mixture • “Hetero” means different • Consists of visibly different substances or phases (solid, liquid, gas) • A suspension is a special type of heterogeneous mixture of larger particles that eventually settle • Example: Trail Mix Notice the visibly different substances

Homogeneous Mixture • “Homo” means the same • Has the same uniform appearance and composition throughout; maintain one phase (solid, liquid, gas) • Commonly referred to as solutions • Example: Salt Water Notice the uniform appearance
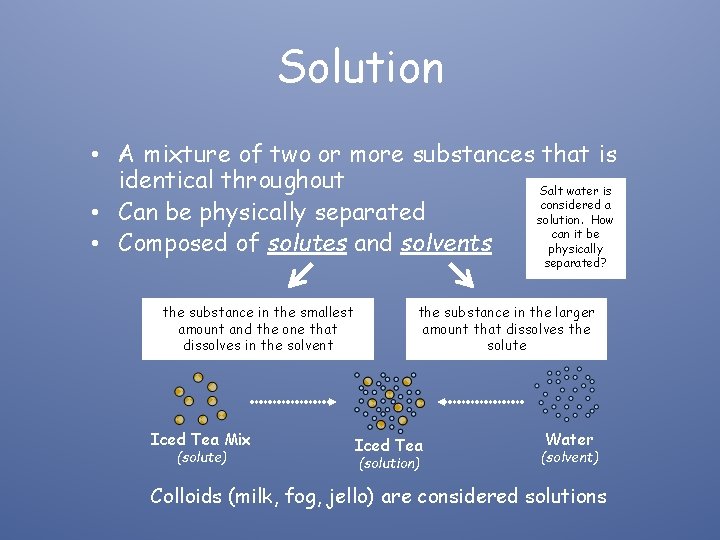
Solution • A mixture of two or more substances that is identical throughout Salt water is considered a • Can be physically separated solution. How can it be • Composed of solutes and solvents physically separated? the substance in the smallest amount and the one that dissolves in the solvent Iced Tea Mix (solute) the substance in the larger amount that dissolves the solute Iced Tea (solution) Water (solvent) Colloids (milk, fog, jello) are considered solutions
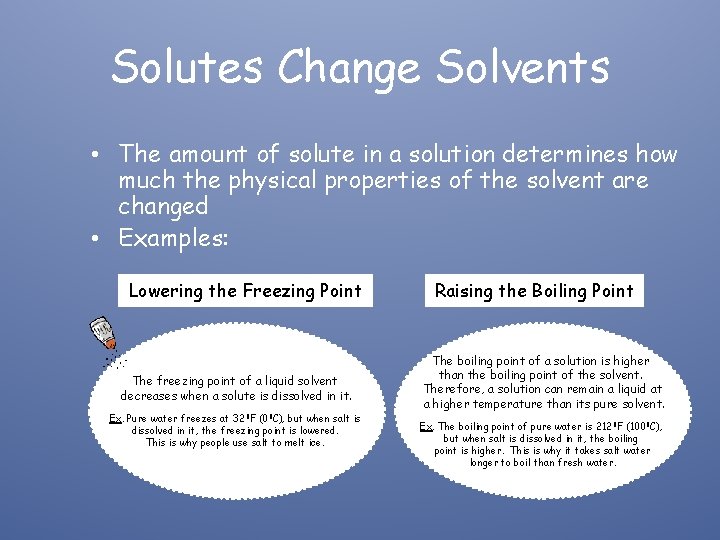
Solutes Change Solvents • The amount of solute in a solution determines how much the physical properties of the solvent are changed • Examples: Lowering the Freezing Point The freezing point of a liquid solvent decreases when a solute is dissolved in it. Ex. Pure water freezes at 32 0 F (00 C), but when salt is dissolved in it, the freezing point is lowered. This is why people use salt to melt ice. Raising the Boiling Point The boiling point of a solution is higher than the boiling point of the solvent. Therefore, a solution can remain a liquid at a higher temperature than its pure solvent. Ex. The boiling point of pure water is 2120 F (1000 C), but when salt is dissolved in it, the boiling point is higher. This is why it takes salt water longer to boil than fresh water.
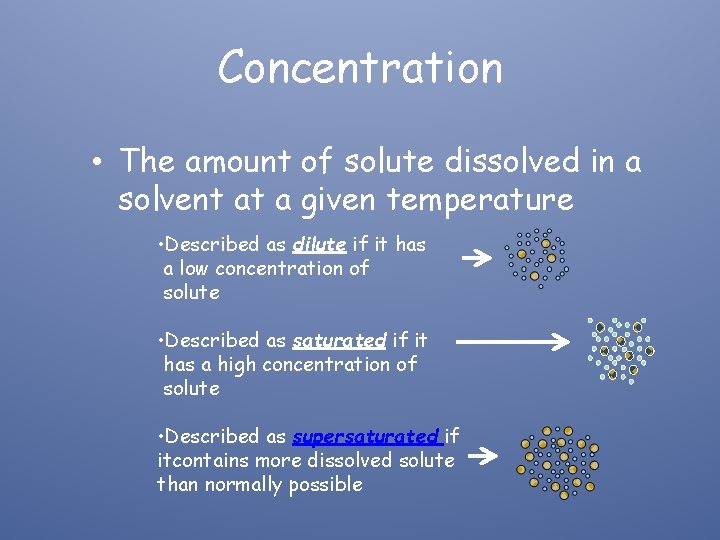
Concentration • The amount of solute dissolved in a solvent at a given temperature • Described as dilute if it has a low concentration of solute • Described as saturated if it has a high concentration of solute • Described as supersaturated if itcontains more dissolved solute than normally possible
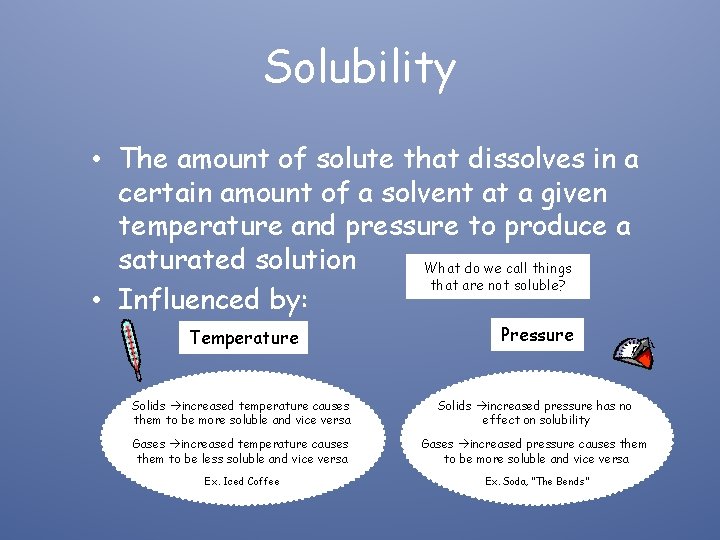
Solubility • The amount of solute that dissolves in a certain amount of a solvent at a given temperature and pressure to produce a saturated solution What do we call things that are not soluble? • Influenced by: Temperature Pressure Solids increased temperature causes them to be more soluble and vice versa Solids increased pressure has no effect on solubility Gases increased temperature causes them to be less soluble and vice versa Gases increased pressure causes them to be more soluble and vice versa Ex. Iced Coffee Ex. Soda, “The Bends”

Review Solutions • Can you see two parts in solutions or are they mixed together so well you only see one thing? – you only see one thing • Are solutions mixtures or pure substances? – Mixtures • What kind of states can a solution be? – Solid, liquid, or gas • What are the two “s” words that every solution must have? – A solute and a solvent

In a salt water solution… • Is salt the solute or the solvent? – Solute • Is water the solute or the solvent? – Solvent • What does the solute do? – Gets dissolved • What does the solvent do? – Does the dissolving
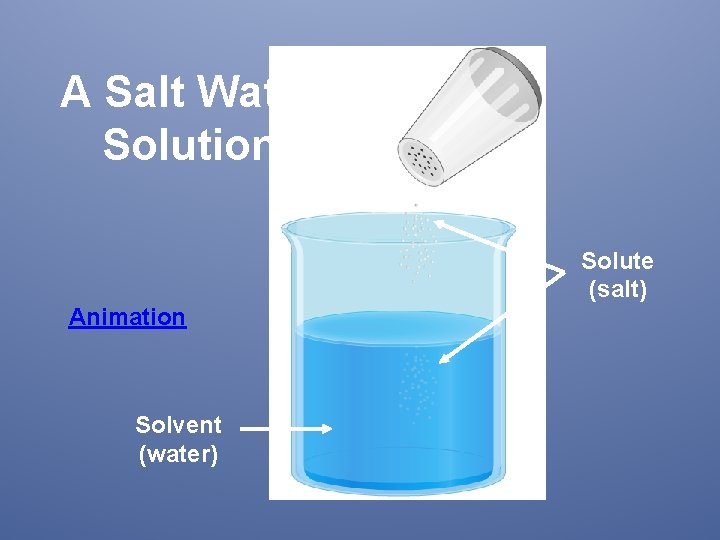
A Salt Water Solution Solute (salt) Animation Solvent (water)
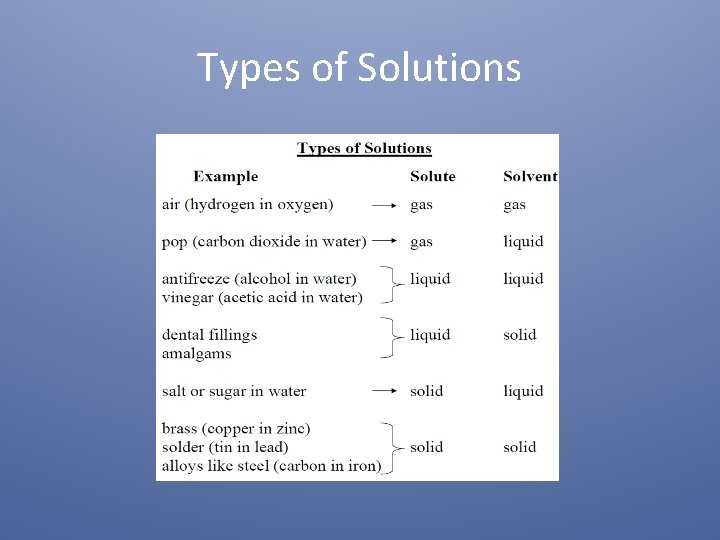
Types of Solutions

Solutions What is the Solute and what is the solvent? Label Each. 1. Cigarette Smoke and Air 2. Caffeine and Water (Cup of Coffee) solute 3. Water and Oxygen (Water in a Fish Tank) 4. Carbon Dioxide and Sugar Water (Sealed Can of Pop) 5. Oxygen and Nitrogen (Air) 6. Minerals and Water (Hard Water) 7. Water and Sugar (Maple Syrup) 8. Acetic Acid and Water (Vinegar) 9. Salt and Water (Ocean Water) 10. Make your own solvent

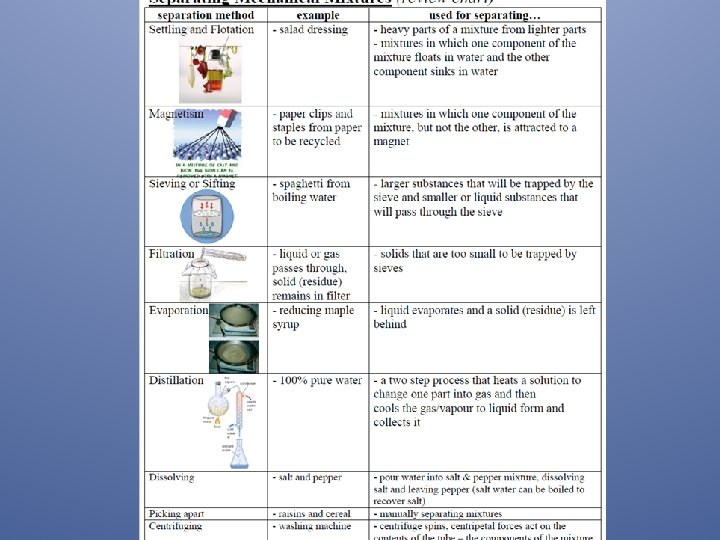
Separating Mixtures & Solutions
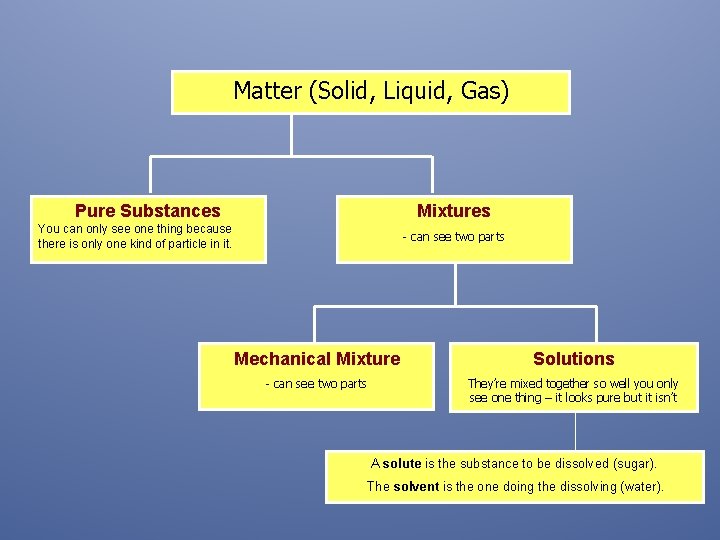
Matter (Solid, Liquid, Gas) Pure Substances Mixtures You can only see one thing because there is only one kind of particle in it. - can see two parts Mechanical Mixture Solutions - can see two parts They’re mixed together so well you only see one thing – it looks pure but it isn’t A solute is the substance to be dissolved (sugar). The solvent is the one doing the dissolving (water).

Suspensions • Suspensions are mixtures with particles large enough to settle out unless the mixture is constantly stirred or agitated.

Colloids • Colloids are mixtures with particles intermediate in size between solutions and suspensions. • You cannot see the individual particles. • The particles do not separate upon standing. http: //commons. wikimedia. org/wiki/File: Hk. Symphony_of_Lights_3420. jpg

Examples of Colloids • Sol—Solids dispersed in liquids – Example: Paint • Gel—Solid network through a liquid – Example: Gelatin • Liquid Emulsion—Liquids dispersed in liquids – Example: Milk • Foam—Gases dispersed in liquids – Example: Shaving cream • Solid Aerosol—Solids dispersed in gases – Example: Smoke • Liquid Aerosol—Liquids dispersed in gases – Example: Fog • Solid Emulsion—Liquids dispersed in solids – Example: Cheese
- Slides: 19