Types of Mixtures Homogeneous mixtures Uniform composition throughout











- Slides: 14

Types of Mixtures

• Homogeneous mixtures – Uniform composition throughout – Examples: salt water, sugar-water, metal alloys • Heterogeneous mixtures – NOT a uniform composition. – May be obvious to the naked eye, or must be viewed under a microscope – Examples: dirt, milk, bread

Types of Mixtures • Solutions – Homogeneous mixtures of two or more substances in a single phase. Small dissolved particles • Suspensions – Heterogeneous mixtures in with large dissolved particles. • Colloids – Heterogeneous mixtures with particles intermediate in size between solutions and suspensions

Solutions • A solution is formed when one substance dissolves in one another. • A substance which can dissolve is called soluble. • The solvent is the substance into which it dissolves. • When Na. Cl dissolves in water, the Na. Cl is the solute and the water is the solvent

Solutions • In a solution, the dissolved particles of the solute are so small that they can’t be seen. – The particles are the size of atoms, molecules, and ions. • The solute doesn’t settle out. • When the solution is poured through filter paper, the entire solution passes through.

Solutions • Solutions don’t have to be liquids. They can be any combination of solid/liquid/gas. – Air – Soda – Salt water – Metal alloy – Ethanol in water – Amalgam

Suspensions • In a suspension, the particles are so large that they settle out unless the mixture is constantly stirred or agitated. • Can be separated by pouring through a filter.

Colloids • Intermediate sized particles are dispersed throughout the dispersing medium. • The particles are small enough that they remain suspended in the solvent. • These mixtures can’t be separated by normal filtration. • Can be any combination of gas/liquid/solid

Types of colloids • • Sol Gel Liquid emulsion Foam Solid aerosol Liquid aerosol Solid emulsion

• It is easy to determine if a mixture is a suspension – let it sit and if the particles settle out, it is a suspension. • The difference between colloids and solutions may be more difficult to determine. – The colloid particles may only be slightly larger than those of a solution, causing the mixture to appear homogeneous

Tyndall Effect • In a colloid, the particles are large enough that they will scatter light • If you shine a beam of light through a colloid, the light beam is visible due to the scattering of the light. • Shining a beam of light through a solution does not yield the same effect.


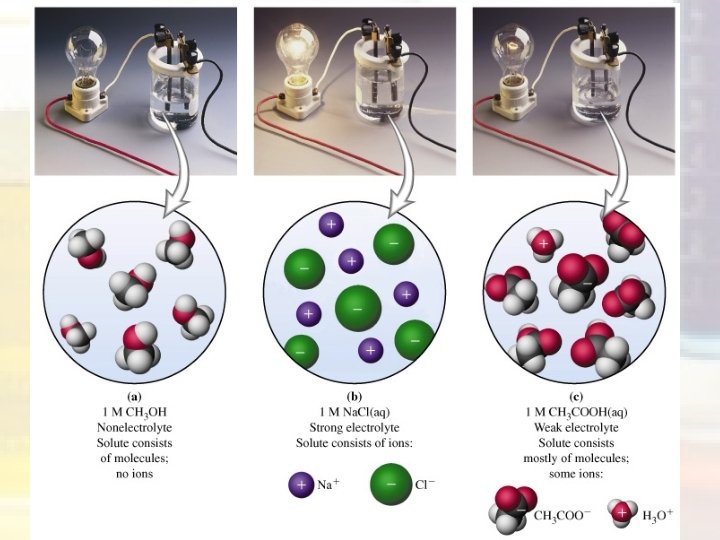
Electrolytes • A substance that dissolves in water and separates into ions in the solution is called an electrolyte. – The solution will conduct electricity – Examples: salt, hydrochloric acid, vinegar • A substance that dissolves in water and DOES NOT separate into ions (it remains as molecules) is a non-electrolyte – The solution will not conduct electricity – Examples: sugar, kerosene
