Types of Matter Types of Matter All the

- Slides: 12

Types of Matter

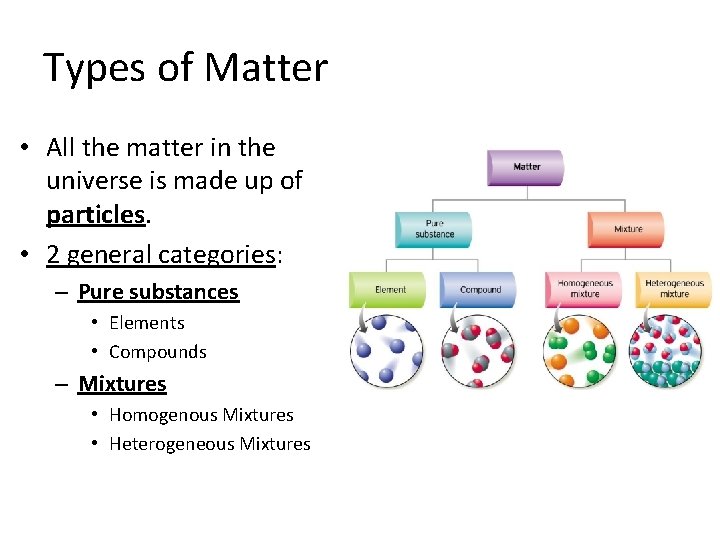
Types of Matter • All the matter in the universe is made up of particles. • 2 general categories: – Pure substances • Elements • Compounds – Mixtures • Homogenous Mixtures • Heterogeneous Mixtures

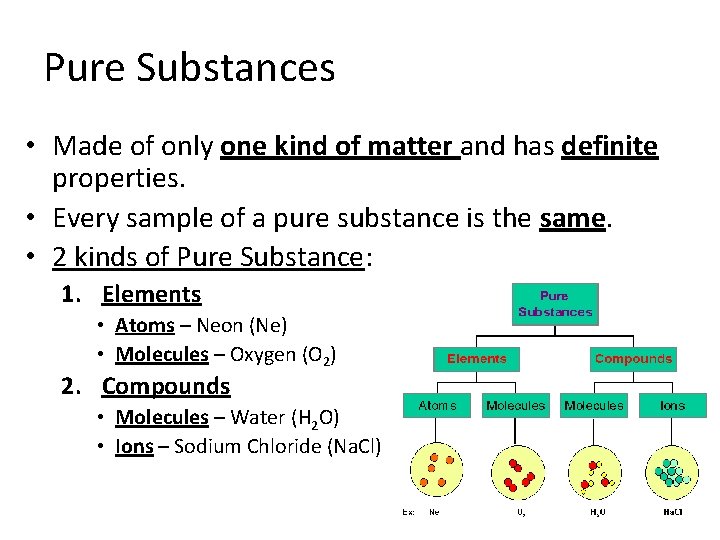
Pure Substances • Made of only one kind of matter and has definite properties. • Every sample of a pure substance is the same. • 2 kinds of Pure Substance: 1. Elements • Atoms – Neon (Ne) • Molecules – Oxygen (O 2) 2. Compounds • Molecules – Water (H 2 O) • Ions – Sodium Chloride (Na. Cl)

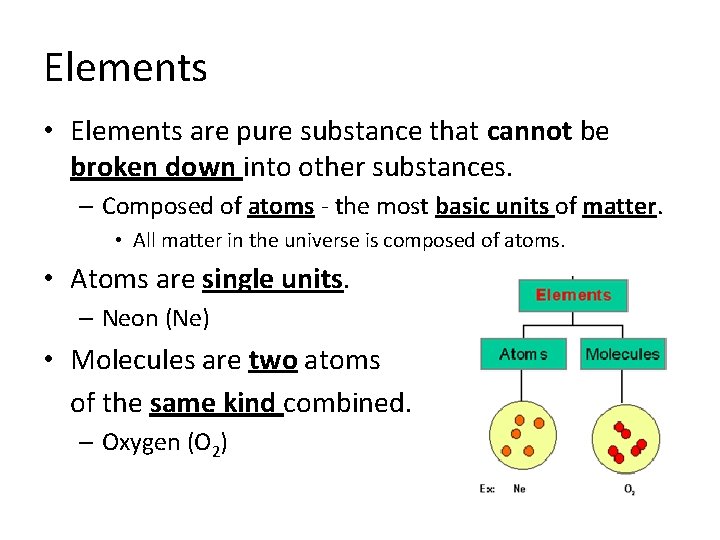
Elements • Elements are pure substance that cannot be broken down into other substances. – Composed of atoms - the most basic units of matter. • All matter in the universe is composed of atoms. • Atoms are single units. – Neon (Ne) • Molecules are two atoms of the same kind combined. – Oxygen (O 2)

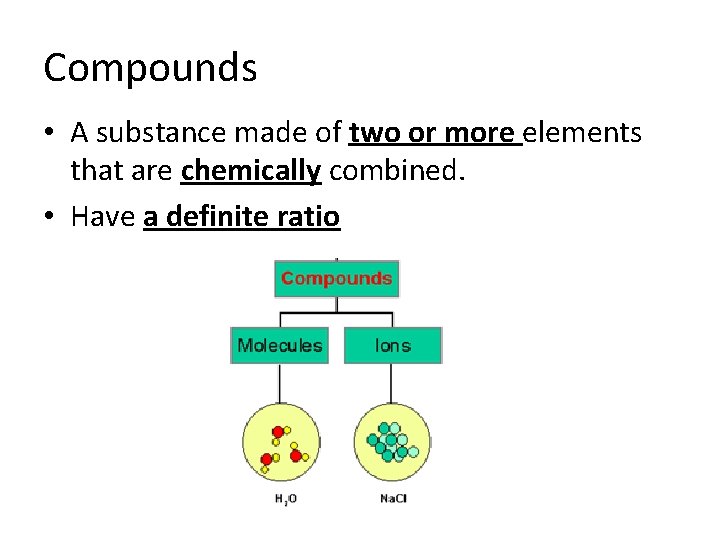
Compounds • A substance made of two or more elements that are chemically combined. • Have a definite ratio

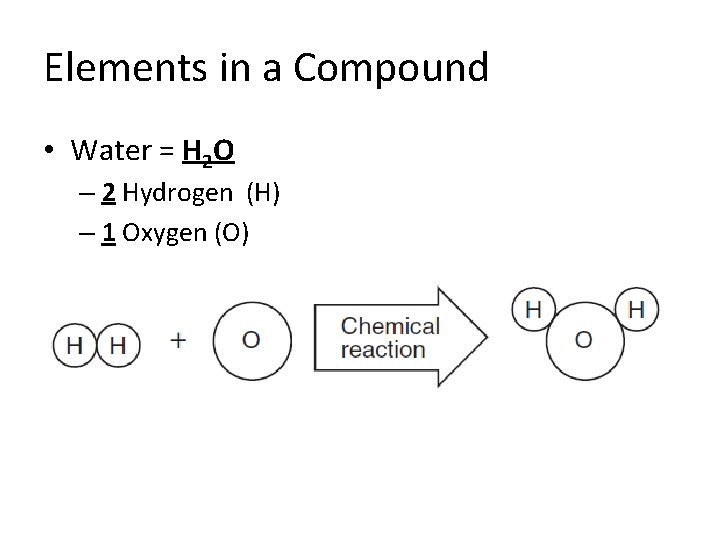
Elements in a Compound • Water = H 2 O – 2 Hydrogen (H) – 1 Oxygen (O)

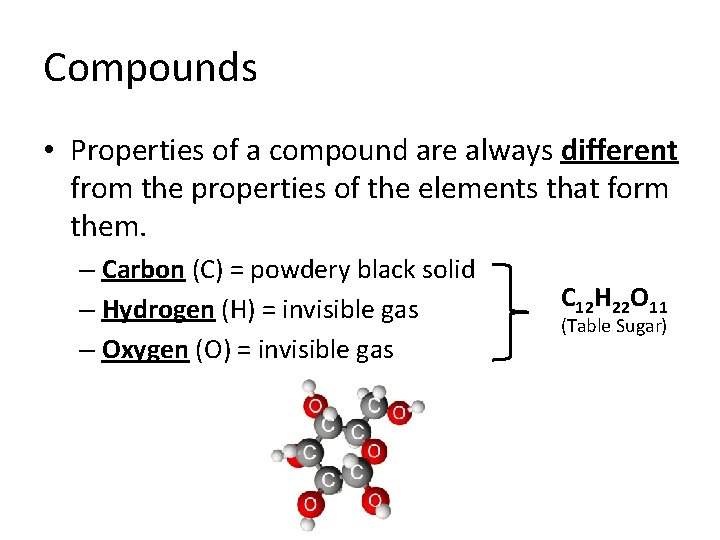
Compounds • Properties of a compound are always different from the properties of the elements that form them. – Carbon (C) = powdery black solid – Hydrogen (H) = invisible gas – Oxygen (O) = invisible gas C 12 H 22 O 11 (Table Sugar)

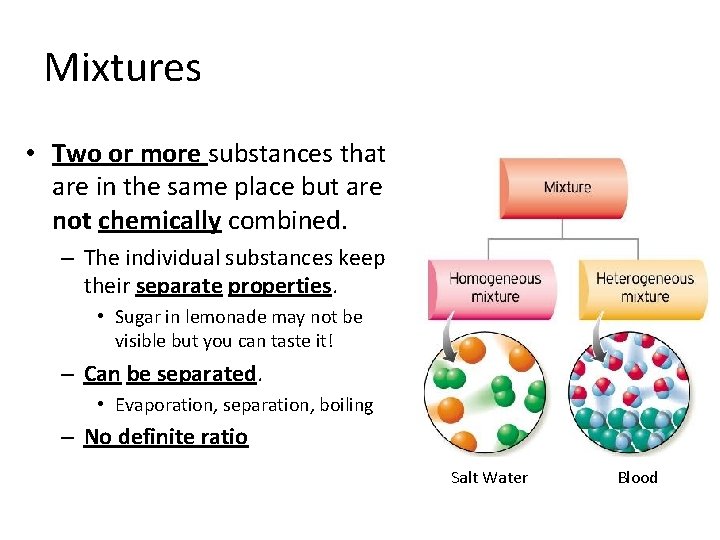
Mixtures • Two or more substances that are in the same place but are not chemically combined. – The individual substances keep their separate properties. • Sugar in lemonade may not be visible but you can taste it! – Can be separated. • Evaporation, separation, boiling – No definite ratio Salt Water Blood

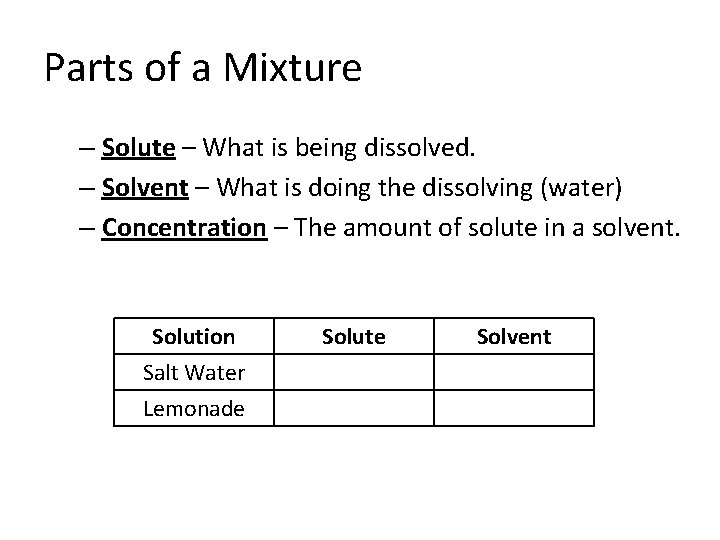
Parts of a Mixture – Solute – What is being dissolved. – Solvent – What is doing the dissolving (water) – Concentration – The amount of solute in a solvent. Solution Salt Water Lemonade Solute Salt Lemonade Mix Solvent Water

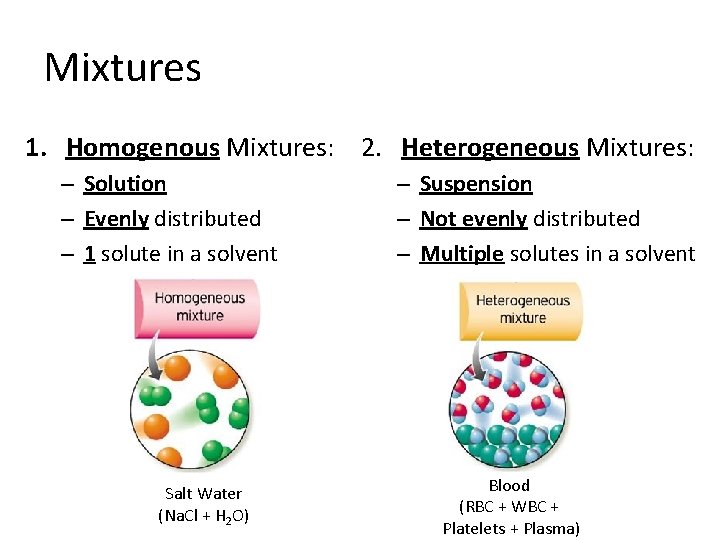
Mixtures 1. Homogenous Mixtures: 2. Heterogeneous Mixtures: – Solution – Evenly distributed – 1 solute in a solvent Salt Water (Na. Cl + H 2 O) – Suspension – Not evenly distributed – Multiple solutes in a solvent Blood (RBC + WBC + Platelets + Plasma)

Solubility • How well a type of matter can dissolve in a solvent is called solubility. • Two factors affect the rate at which solutes dissolve in solvents: 1. Temperature • The higher the temperature of the liquid solvent, the more solute can be dissolved. • The higher the temperature of a gas solvent, the less solute can be dissolved. 2. Pressure • The greater the pressure of the solvent, the more solute can be dissolved.

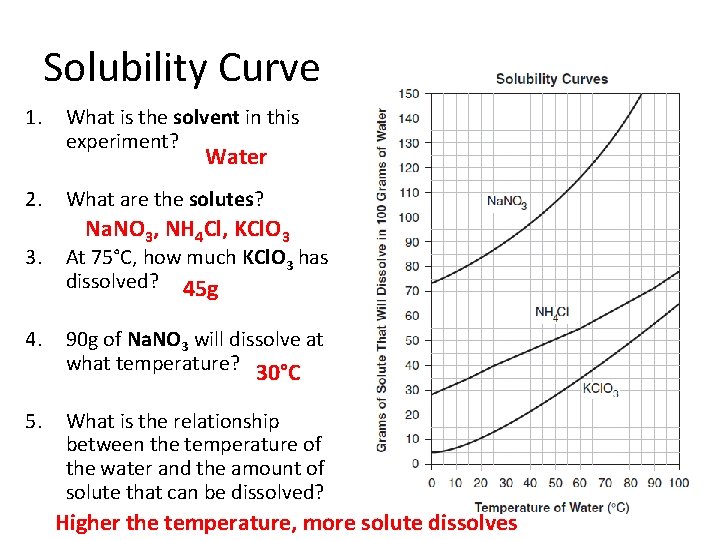
Solubility Curve 1. What is the solvent in this experiment? Water 2. What are the solutes? Na. NO 3, NH 4 Cl, KCl. O 3 3. At 75°C, how much KCl. O 3 has dissolved? 45 g 4. 90 g of Na. NO 3 will dissolve at what temperature? 30°C 5. What is the relationship between the temperature of the water and the amount of solute that can be dissolved? Higher the temperature, more solute dissolves