Types of Matter Notes Vocabulary 12 13 1

Types of Matter Notes Vocabulary: 12. 13. 1. chemistry 14. 2. matter 15. 3. pure substance 4. representative particle 16. 17. 5. element 6. symbol 7. compound 8. formula 9. molecule/formula unit 10. Law of Definite Composition 11. mixture heterogeneous mixture homogeneous mixture solution solid liquid gas “No. 8”, Jackson Pollack, 1949
What is chemistry? Chemistry is the study of matter. Anything that has mass and takes up space.
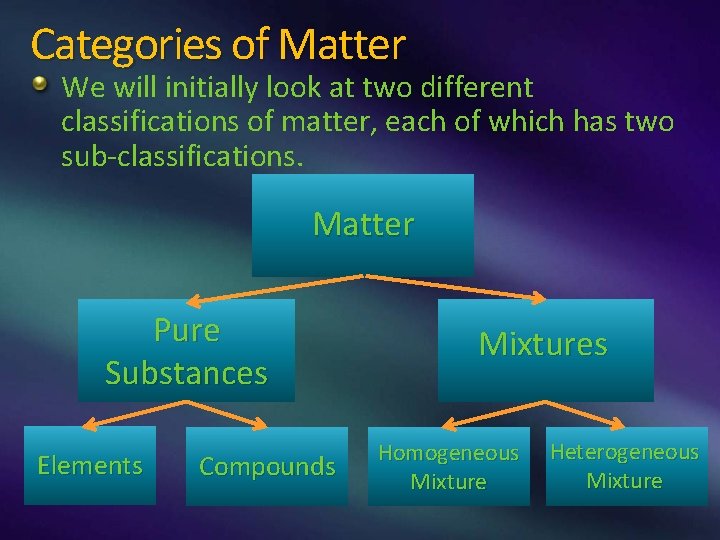
Categories of Matter We will initially look at two different classifications of matter, each of which has two sub-classifications. Matter Pure Substances Elements Compounds Mixtures Homogeneous Mixture Heterogeneous Mixture

Definition Time! Pure substance: a substance containing only one type of representative particle. Representative particle: the smallest part of a substance containing all the properties of the substance.

Element A pure substance made of ONLY ONE TYPE OF ATOM. Representative particle: the atom Found on the periodic table. Has a symbol (first letter is upper case and second letter, if any, is lower case. )
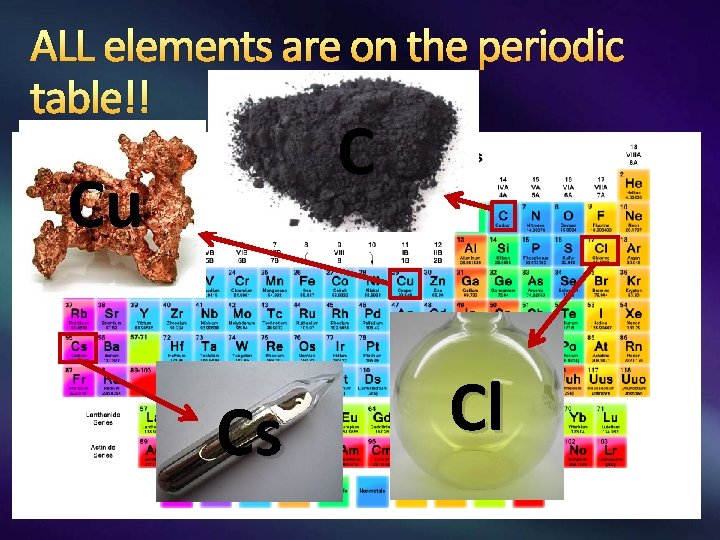
ALL elements are on the periodic table!! C Cu Cs Cl
Compound A pure substance that is made from the atoms of two or more elements that are chemically combined. Has a formula containing the symbols of two or more elements. Follows the Law of Definite Composition. Representative particle: molecules or formula units. Components can only be chemically separated.

Definition Law of Definite Composition: Every compound has a fixed number of atoms of its component elements (e. g. , water always has 2 hydrogen atoms and one oxygen atom in each molecule. )
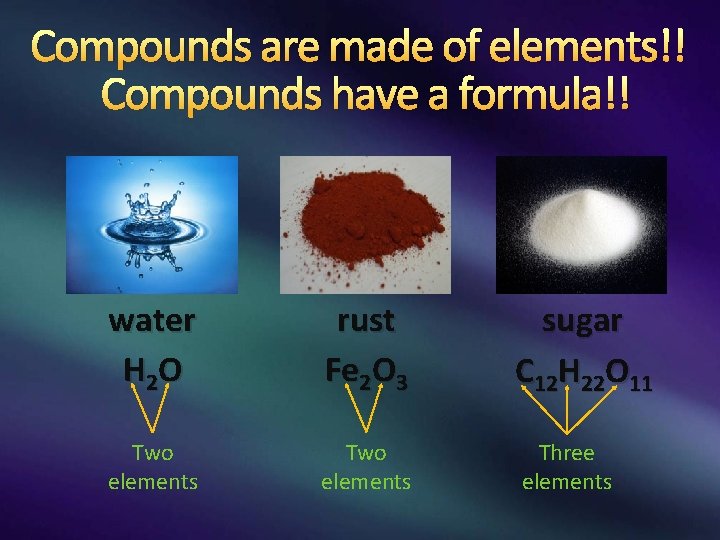
Compounds are made of elements!! Compounds have a formula!! water H 2 O rust Fe 2 O 3 sugar C 12 H 22 O 11 Two elements Three elements

Mixture Two or more types of matter that are blended, but not chemically combined. Each substance in the mixture maintains its identity and properties. There is no representative particle for the mixture, only the individual components of the mixture. Components can be physically separated.

Mixtures No symbol or formula!

Heterogeneous Mixture Hetero: Different A mixture that is not uniform throughout on a particle level. Easy to see the different components so obviously it is heterogeneous. Can not see through the chocolate milk, so particles are not uniform throughout, and therefore heterogeneous.

Homogeneous Mixture Homo: Same A mixture which is uniform throughout on a particle level. Also called solutions Kool Aid: Can not see the components and clear, so homogeneous. Salt Water: Tough one. You have to be told that the water has salt in it. You can’t see the salt, so homogeneous. White Gold: Tough one. You have to be told that is made of silver and gold mixed together (called an “alloy, ” a mixture of metals). Homogeneous.
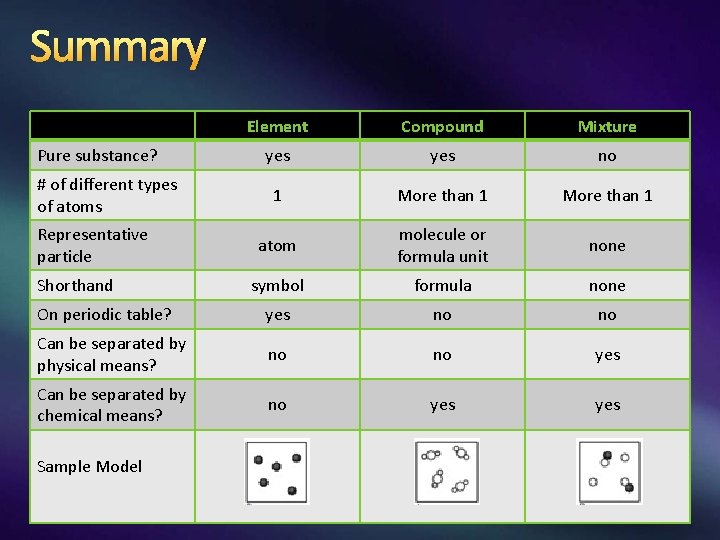
Summary Element Compound Mixture yes no 1 More than 1 atom molecule or formula unit none symbol formula none On periodic table? yes no no Can be separated by physical means? no no yes Can be separated by chemical means? no yes Pure substance? # of different types of atoms Representative particle Shorthand Sample Model

Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?

Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?
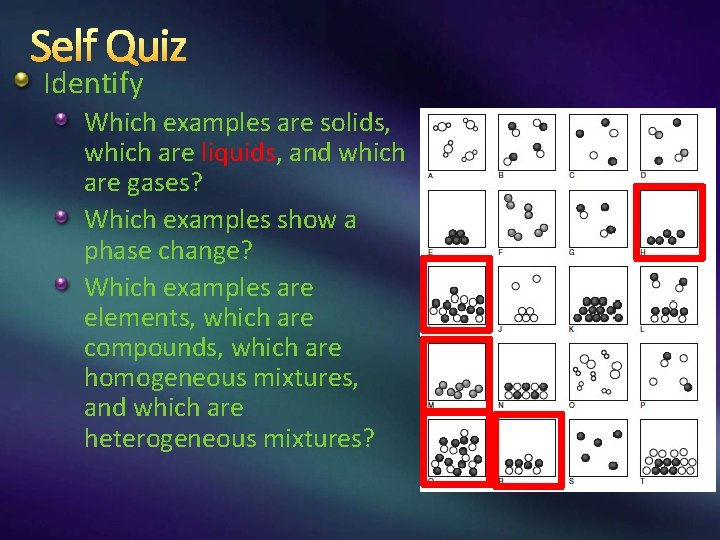
Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?

Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?

Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?
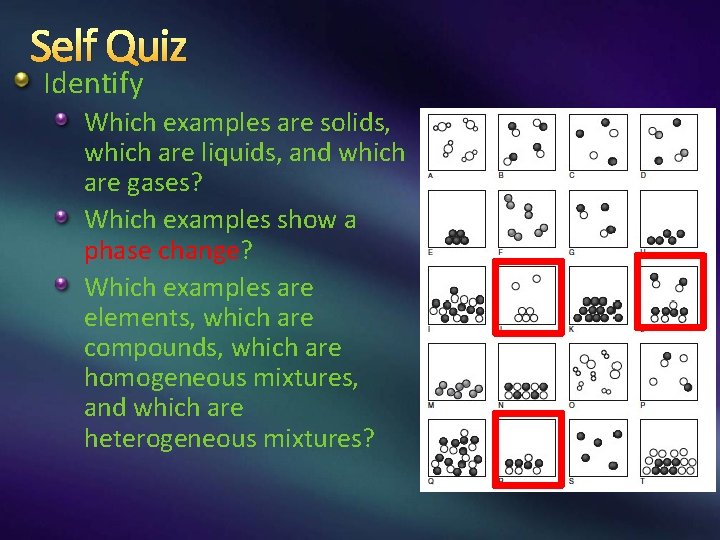
Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?

Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?
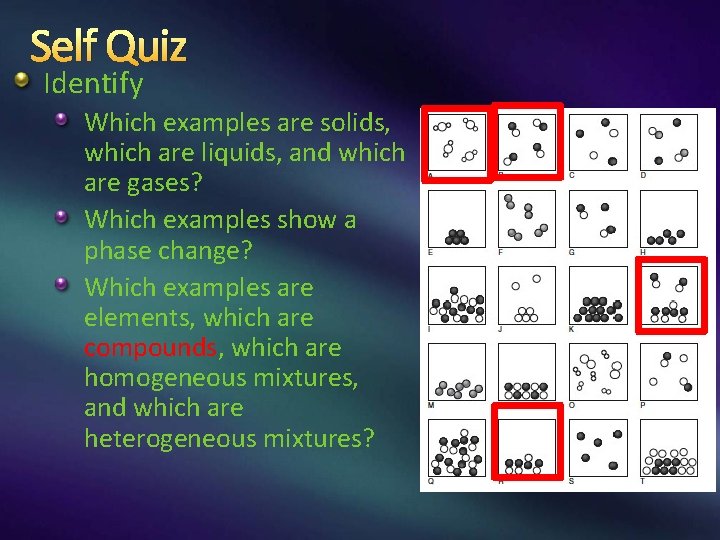
Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?
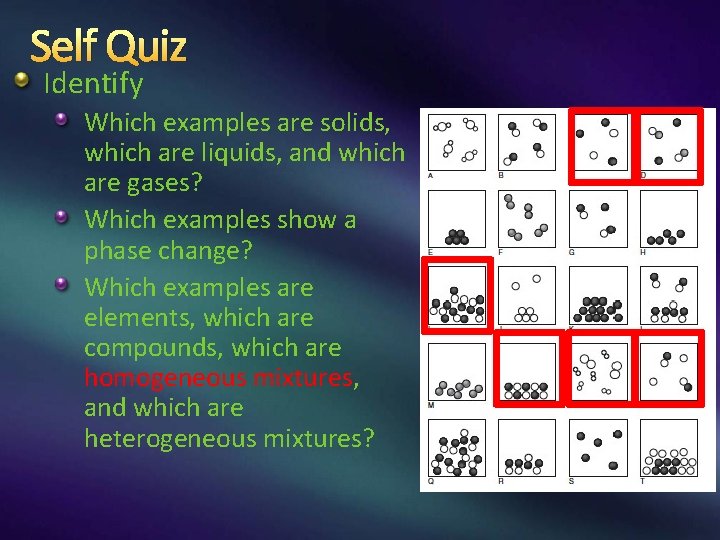
Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?

Self Quiz Identify Which examples are solids, which are liquids, and which are gases? Which examples show a phase change? Which examples are elements, which are compounds, which are homogeneous mixtures, and which are heterogeneous mixtures?
- Slides: 24