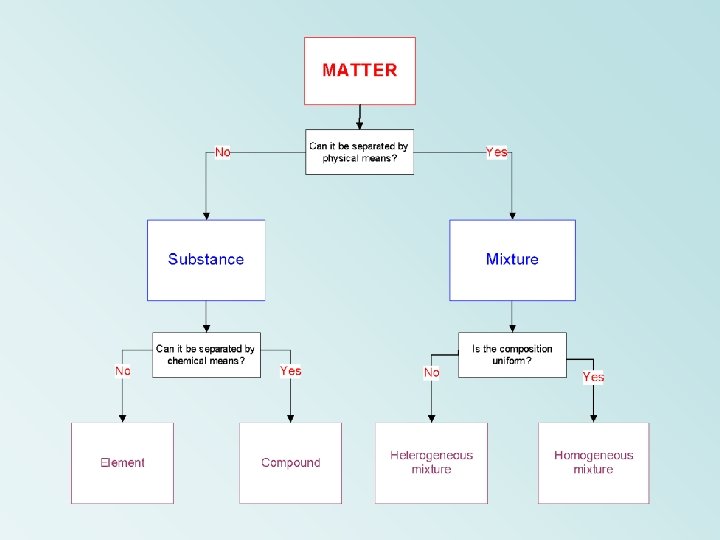
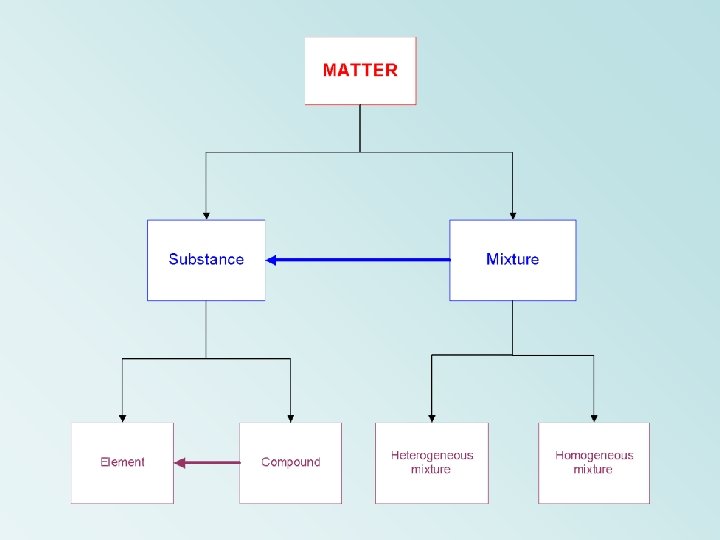
Types of Matter can be divided into one
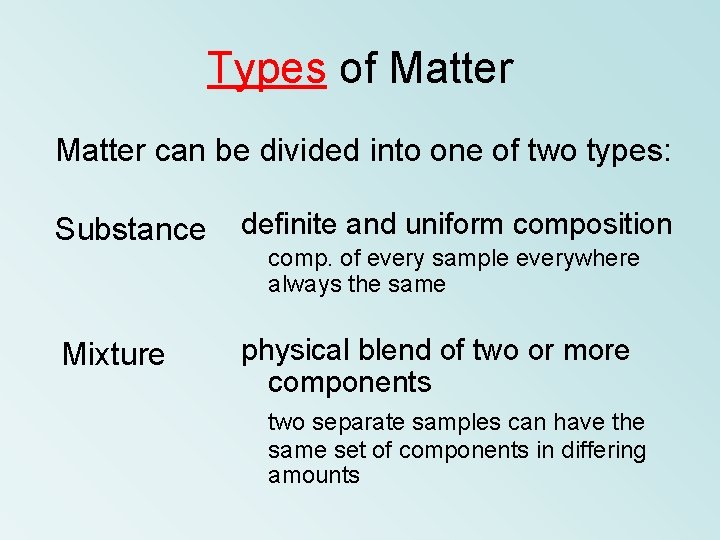
Types of Matter can be divided into one of two types: Substance definite and uniform composition Mixture physical blend of two or more components comp. of every sample everywhere always the same two separate samples can have the same set of components in differing amounts
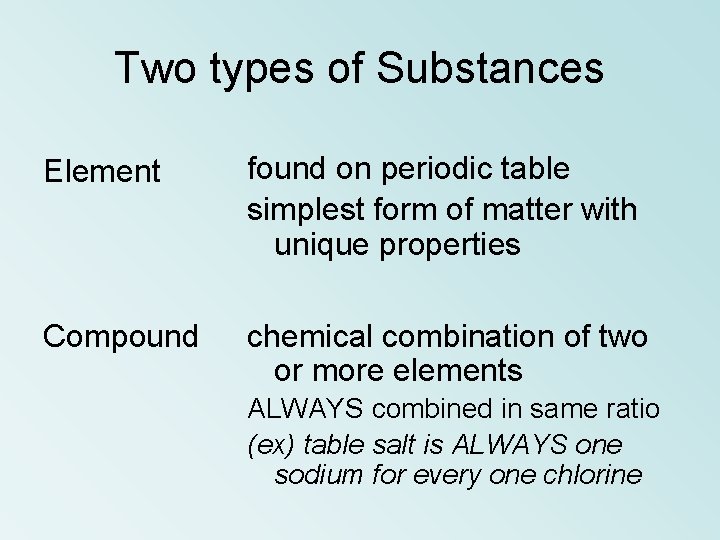
Two types of Substances Element found on periodic table simplest form of matter with unique properties Compound chemical combination of two or more elements ALWAYS combined in same ratio (ex) table salt is ALWAYS one sodium for every one chlorine

Two types of Mixtures Homogeneous given sample is uniform throughout, evenly mixed (ex) Sugar dissolved in water Heterogeneous NOT uniform (ex) Rocky Road ice-cream Solutions are a type of homogeneous mixture as are alloys
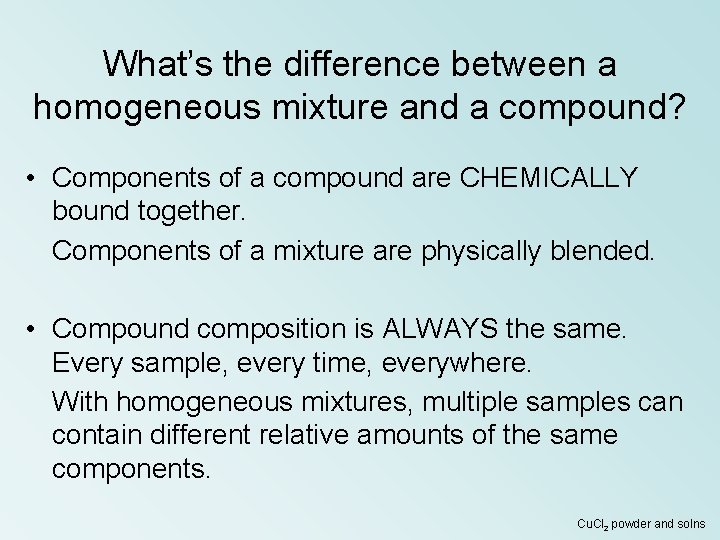
What’s the difference between a homogeneous mixture and a compound? • Components of a compound are CHEMICALLY bound together. Components of a mixture are physically blended. • Compound composition is ALWAYS the same. Every sample, every time, everywhere. With homogeneous mixtures, multiple samples can contain different relative amounts of the same components. Cu. Cl 2 powder and solns
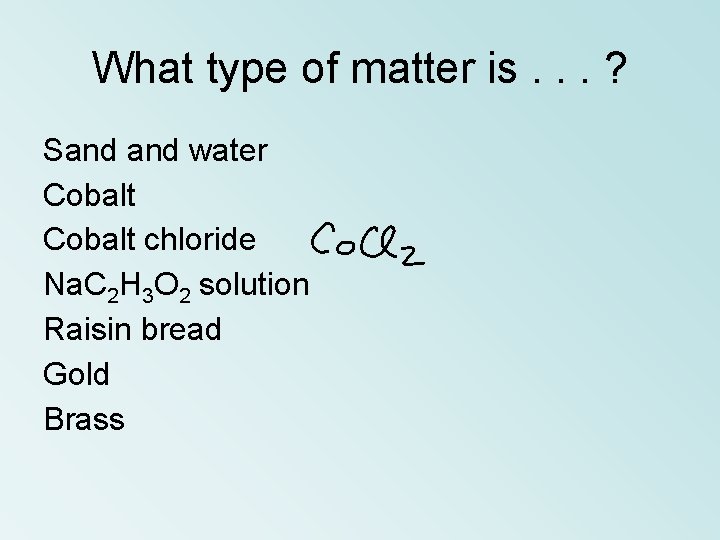
What type of matter is. . . ? Sand water Cobalt chloride Na. C 2 H 3 O 2 solution Raisin bread Gold Brass
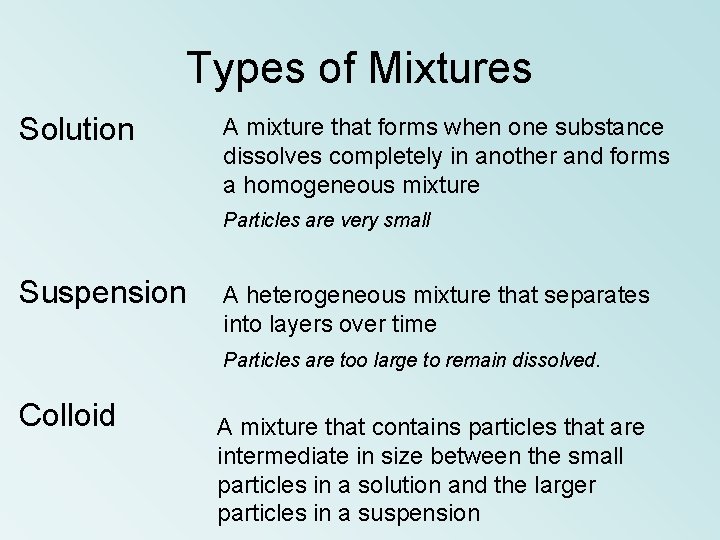
Types of Mixtures Solution A mixture that forms when one substance dissolves completely in another and forms a homogeneous mixture Particles are very small Suspension A heterogeneous mixture that separates into layers over time Particles are too large to remain dissolved. Colloid A mixture that contains particles that are intermediate in size between the small particles in a solution and the larger particles in a suspension
Describing Matter • Physical properties • Physical changes • Chemical properties • Chemical changes

Physical Properties a quality or condition of a substance that can be observed or measured without changing the composition (ex) viscosity, conductivity, malleability, hardness, melting point, boiling point, density

Viscosity resistance to flowing Conductivity ability to allow heat/electricity to flow ability of a solid to be hammered without shattering Malleability Hardness (scratch test) MP / BP temperature at which phase change occurs Density mass / volume
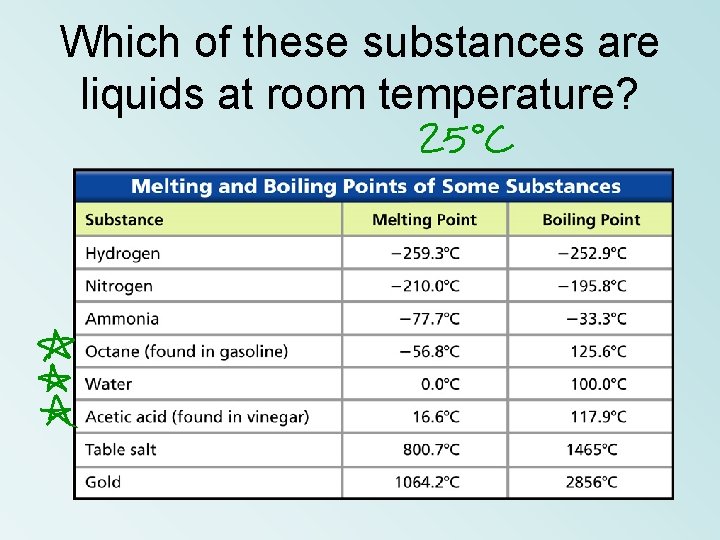
Which of these substances are liquids at room temperature?

Physical Changes that alter a substance without changing its composition (ex) Cutting, grinding, bending, freezing, melting, dissolving, cracking, crushing
Chemical Property describes the ability of a substance to undergo a chemical reaction and form new substances Ex: ability of iron to rust Observed only when a substance undergoes a chemical change…. .
Chemical changes Always results in a change in the chemical composition of the substances involved (ex) Rust, rot, decompose, ferment, explode, corrode

“When matter undergoes a chemical change, the composition of the matter changes. When matter undergoes a physical change, the composition of the matter remains the same. ”

Clues for identifying Chem Rxns/Changes 1. Energy is absorbed or given off as heat* or light 2. Change in color* 3. Production of a gas* (see “smoke”, see bubbles in a soln, smell odor) 4. Formation of a precipitate (solid) * Can also just be a physical change
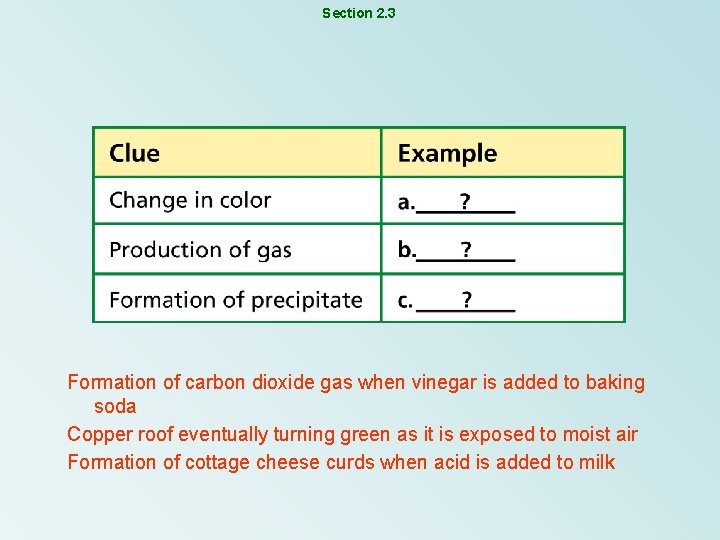
Section 2. 3 Formation of carbon dioxide gas when vinegar is added to baking soda Copper roof eventually turning green as it is exposed to moist air Formation of cottage cheese curds when acid is added to milk
- Slides: 18