Types of K expressions All Keq expressions are


- Slides: 8

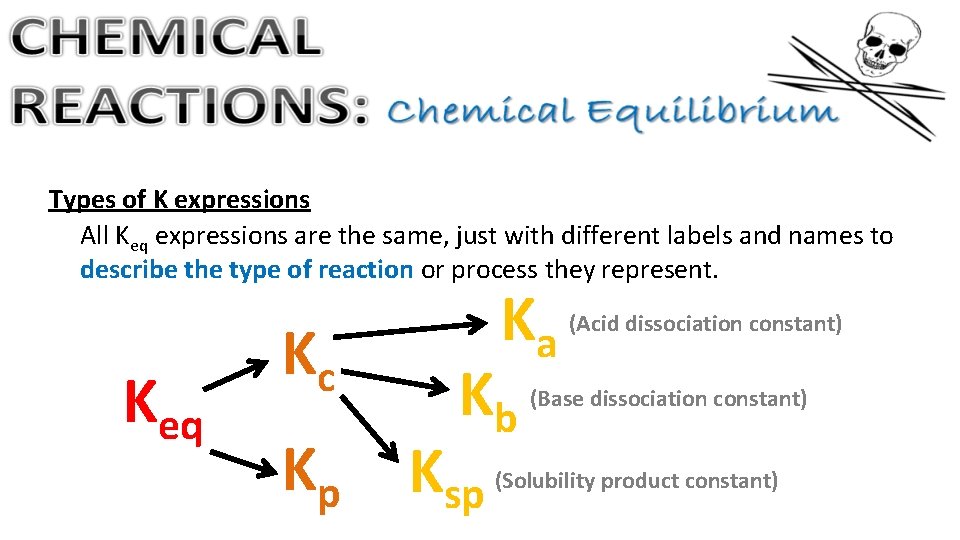
Types of K expressions All Keq expressions are the same, just with different labels and names to describe the type of reaction or process they represent. Keq Kc Kp Ka Kb Ksp (Acid dissociation constant) (Base dissociation constant) (Solubility product constant)

Reaction Quotient: A numerical test to see if a reaction is at eqm or not AND which direction it has to go to get there. Reaction quotient = Q =

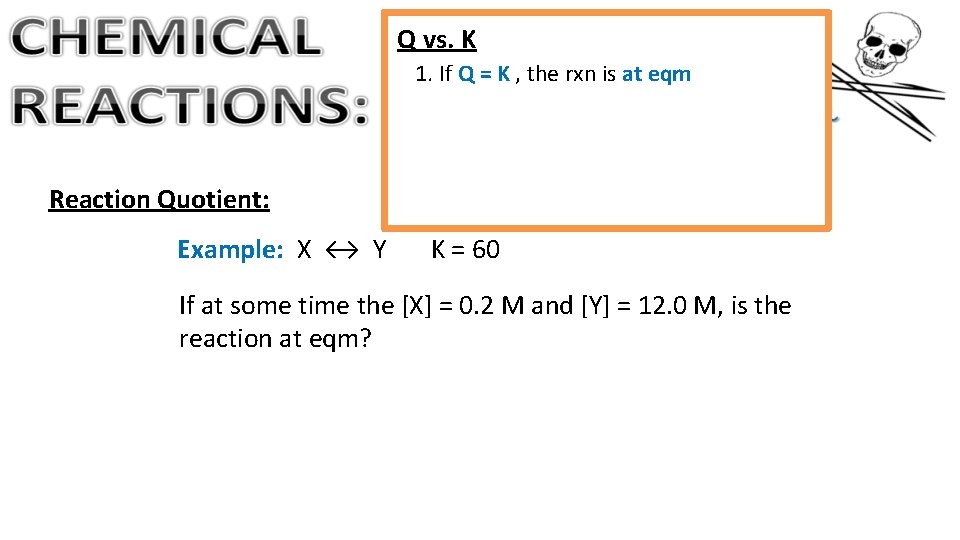
Q vs. K 1. If Q = K , the rxn is at eqm Reaction Quotient: Example: X ↔ Y K = 60 If at some time the [X] = 0. 2 M and [Y] = 12. 0 M, is the reaction at eqm?

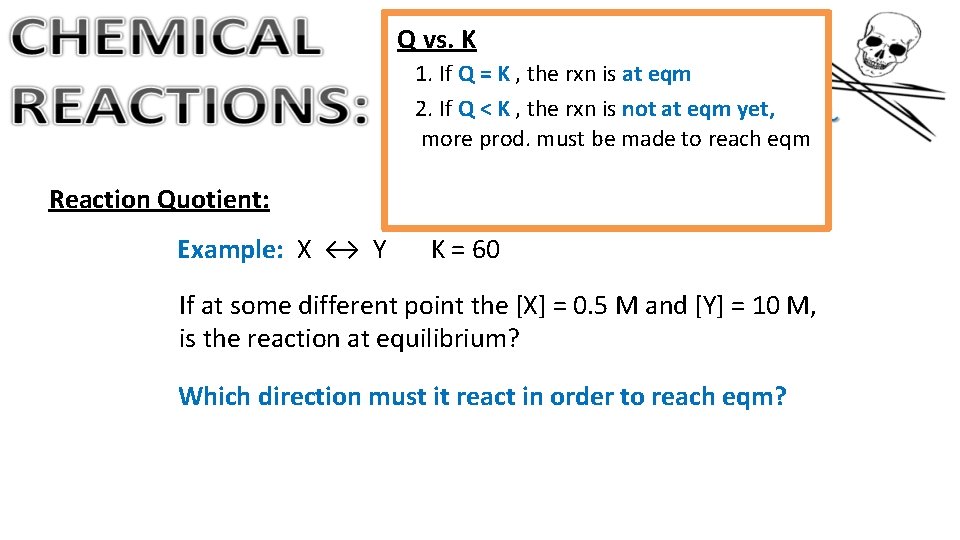
Q vs. K 1. If Q = K , the rxn is at eqm 2. If Q < K , the rxn is not at eqm yet, more prod. must be made to reach eqm Reaction Quotient: Example: X ↔ Y K = 60 If at some different point the [X] = 0. 5 M and [Y] = 10 M, is the reaction at equilibrium? Which direction must it react in order to reach eqm?

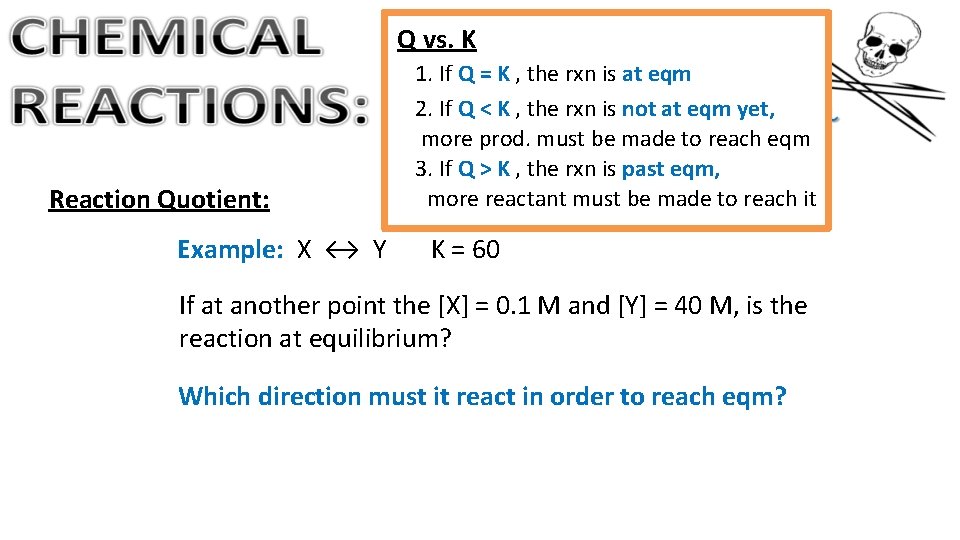
Q vs. K Reaction Quotient: Example: X ↔ Y 1. If Q = K , the rxn is at eqm 2. If Q < K , the rxn is not at eqm yet, more prod. must be made to reach eqm 3. If Q > K , the rxn is past eqm, more reactant must be made to reach it K = 60 If at another point the [X] = 0. 1 M and [Y] = 40 M, is the reaction at equilibrium? Which direction must it react in order to reach eqm?

Example 1: Butane (C 4 H 10) isobutane (C 4 H 10) If the [isobutane] = 4. 0 and [butane] = 3. 0, is the system at equilibrium? Which direction must it proceed to reach equilibrium?

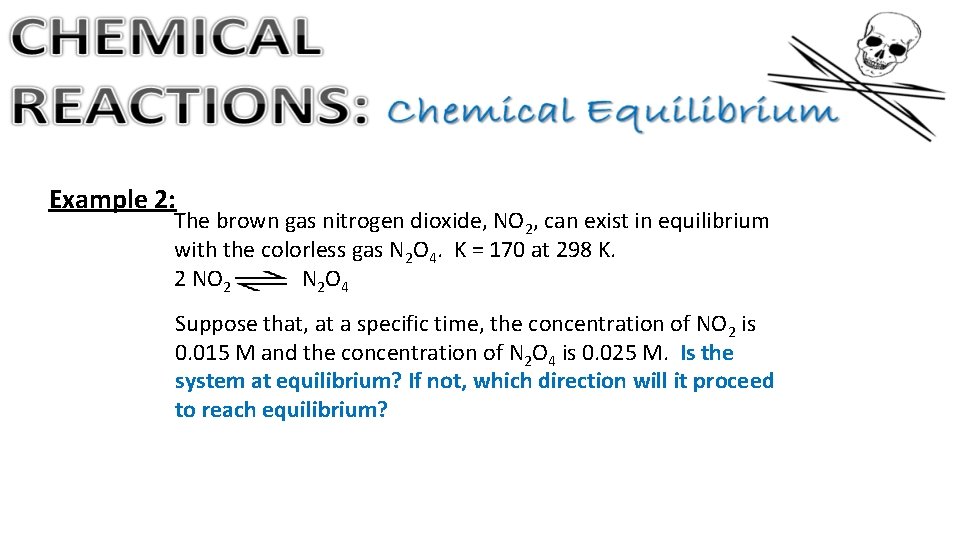
Example 2: The brown gas nitrogen dioxide, NO 2, can exist in equilibrium with the colorless gas N 2 O 4. K = 170 at 298 K. 2 NO 2 N 2 O 4 Suppose that, at a specific time, the concentration of NO 2 is 0. 015 M and the concentration of N 2 O 4 is 0. 025 M. Is the system at equilibrium? If not, which direction will it proceed to reach equilibrium?

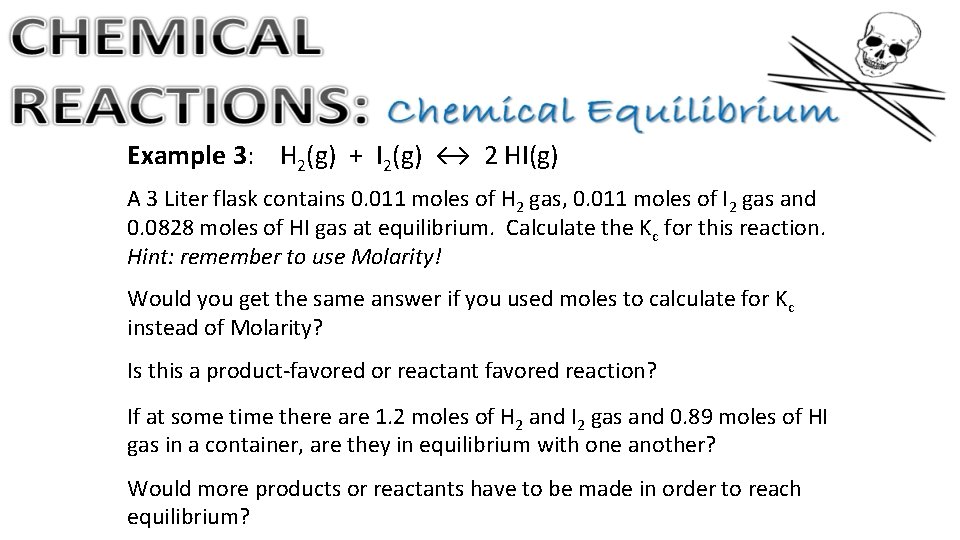
Example 3: H 2(g) + I 2(g) ↔ 2 HI(g) A 3 Liter flask contains 0. 011 moles of H 2 gas, 0. 011 moles of I 2 gas and 0. 0828 moles of HI gas at equilibrium. Calculate the Kc for this reaction. Hint: remember to use Molarity! Would you get the same answer if you used moles to calculate for Kc instead of Molarity? Is this a product-favored or reactant favored reaction? If at some time there are 1. 2 moles of H 2 and I 2 gas and 0. 89 moles of HI gas in a container, are they in equilibrium with one another? Would more products or reactants have to be made in order to reach equilibrium?