Types of hemoglobin BIOCHEMISTRY DR Aqsa Malik Normal
Types of hemoglobin BIOCHEMISTRY DR Aqsa Malik

Normal levels are: �Men: 13. 8 to 17. 2 g/dl �Women: 12. 1 to 15. 1 g/dl �Children: 11 to 16 g/dl �Pregnant women: 11 to 12 g/dl

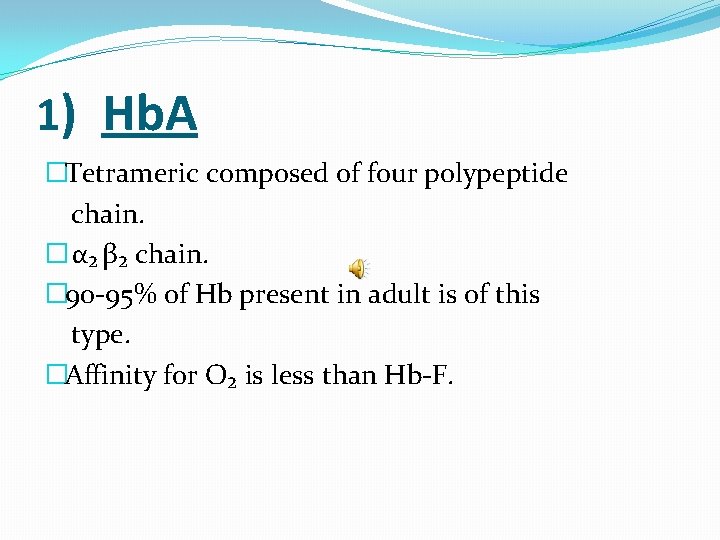
1) Hb. A �Tetrameric composed of four polypeptide chain. � α₂ β₂ chain. � 90 -95% of Hb present in adult is of this type. �Affinity for O₂ is less than Hb-F.

2. Hb. A₂ � � It is a minor component of normal Hb. It appearing shortly before birth. 2% of total Hb. Composed of α₂δ₂ chains.

3. Hb-F �Hb. F is a tetramer consisting of two α chains �identica l to those found in Hb. A, plus two γ chains (α 2γ 2) � The γ chains are members of the β-globin gene family.

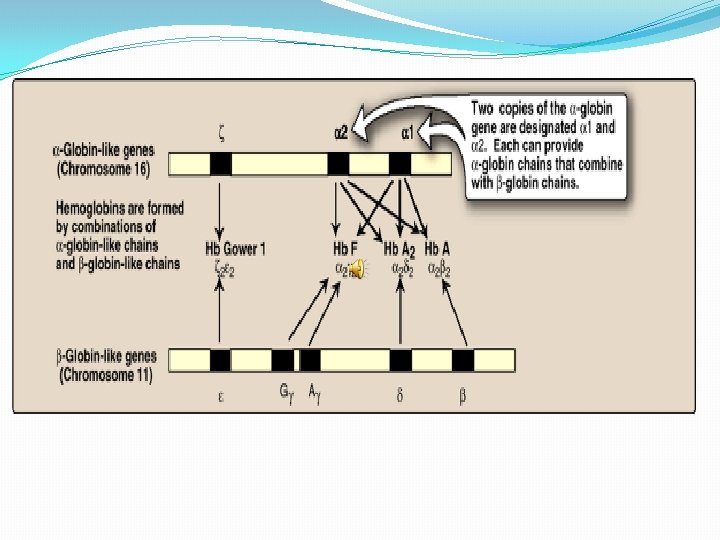
Hb F synthesis during development � In the first few weeks after conception, embryonic hemoglobin (Hb Gower 1), composed of two zeta chains and two epsilon chains, is synthesized by the embryonic yolk sac. �In the fifth week of gestation, the site of globin synthesis shifts , first to the liver and then to the marrow, and the primary product is Hb. F.

�Hb. F is the major hemoglobin found in the fetus and newborn, accounting for about 60% of the total hemoglobin in the RBC during the last months of fetal life. �Hb. A synthesis starts in the bone marrow at about the eighth month of pregnancy and gradually replaces Hb. F. �Hb. F represents less than 1% of the hemoglobin in most adults and is concentrated in RBC known as F cells.

�Binding of 2, 3 -BPG to Hb F: Under physiologic conditions, Hb F has a higher affinity for oxygen than does Hb A, as a result of Hb F's binding only weakly to 2, 3 -BPG �The β-globin chains of Hb F lack some of the positively charged amino acids that are responsible for binding 2, 3 -BPG in the β-globin chains.
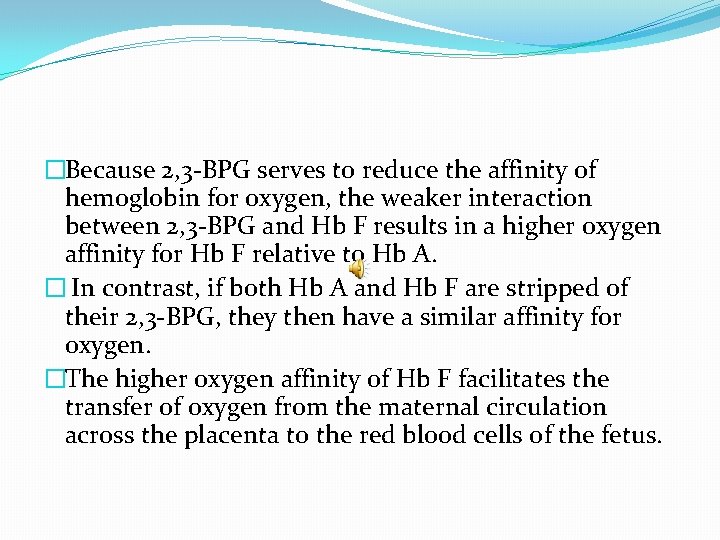
�Because 2, 3 -BPG serves to reduce the affinity of hemoglobin for oxygen, the weaker interaction between 2, 3 -BPG and Hb F results in a higher oxygen affinity for Hb F relative to Hb A. � In contrast, if both Hb A and Hb F are stripped of their 2, 3 -BPG, they then have a similar affinity for oxygen. �The higher oxygen affinity of Hb F facilitates the transfer of oxygen from the maternal circulation across the placenta to the red blood cells of the fetus.

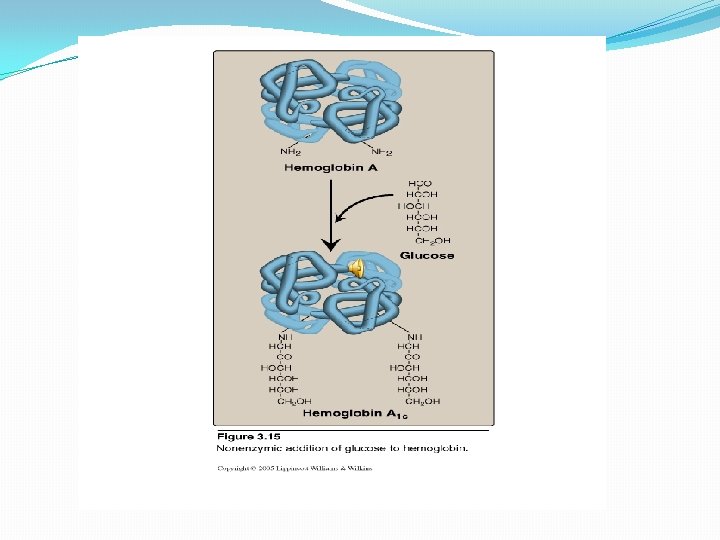
4. Hb. A₁c(glycosylated Hb) Non-enzymic addition of glucose to hemoglobin. � Glucose residue attached to the NH₂ groups of N-terminal valines of the β chains. � In normal individual its concentration is 3 -10% of normal Hb. �In diabetic patient it may be raised to as much as 6 -15% of total Hb. �

Types in humans In the embryo: �Gower 1 (ζ 2ε 2) �Gower 2 (α 2ε 2) �Hemoglobin Portland (ζ 2γ 2)

In the fetus: �Hemoglobin F (α 2γ 2)
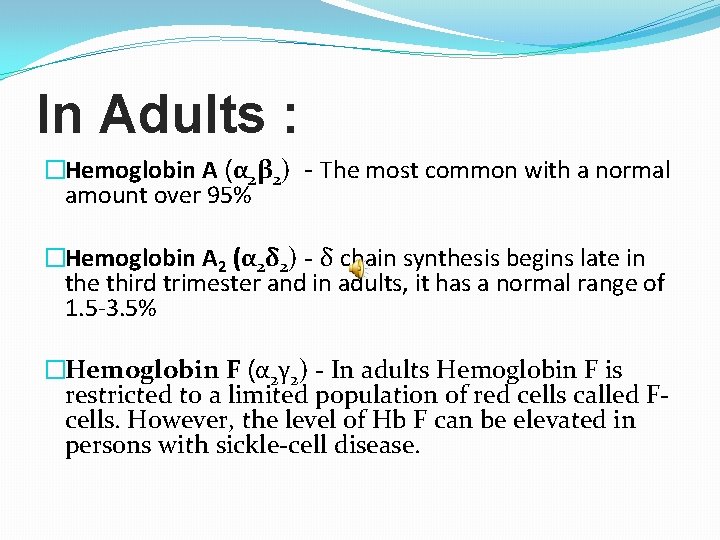
In Adults : �Hemoglobin A (α 2β 2) - The most common with a normal amount over 95% �Hemoglobin A 2 (α 2δ 2) - δ chain synthesis begins late in the third trimester and in adults, it has a normal range of 1. 5 -3. 5% �Hemoglobin F (α 2γ 2) - In adults Hemoglobin F is restricted to a limited population of red cells called Fcells. However, the level of Hb F can be elevated in persons with sickle-cell disease.
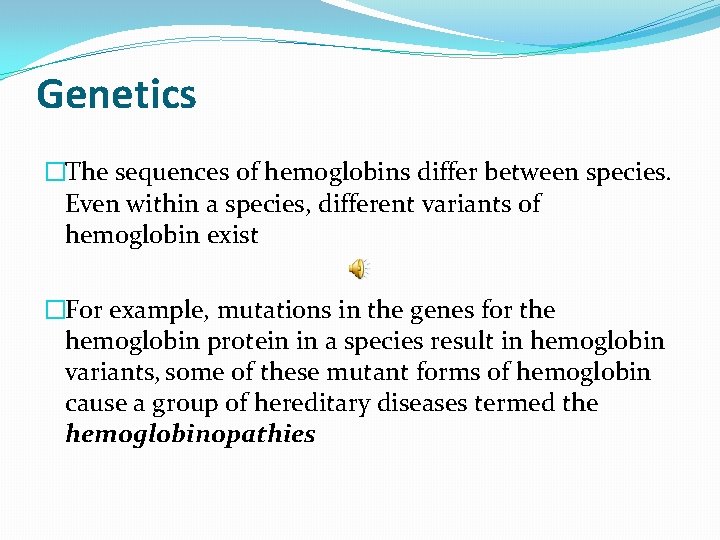
Genetics �The sequences of hemoglobins differ between species. Even within a species, different variants of hemoglobin exist �For example, mutations in the genes for the hemoglobin protein in a species result in hemoglobin variants, some of these mutant forms of hemoglobin cause a group of hereditary diseases termed the hemoglobinopathies

�To understand diseases resulting from genetic alterations in the structure or synthesis of hemoglobins, it is necessary to grasp how the hemoglobin genes, which direct the synthesis of the different globin chains, are structurally organized into gene families and also how they are expressed
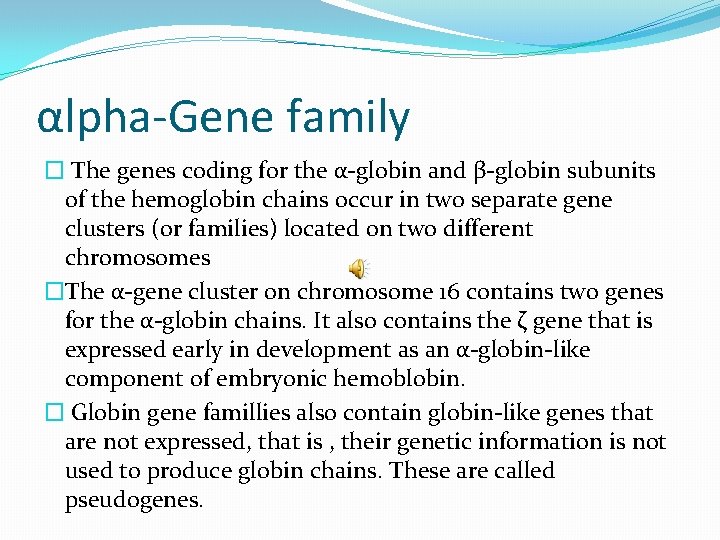
αlpha-Gene family � The genes coding for the α-globin and β-globin subunits of the hemoglobin chains occur in two separate gene clusters (or families) located on two different chromosomes �The α-gene cluster on chromosome 16 contains two genes for the α-globin chains. It also contains the ζ gene that is expressed early in development as an α-globin-like component of embryonic hemoblobin. � Globin gene famillies also contain globin-like genes that are not expressed, that is , their genetic information is not used to produce globin chains. These are called pseudogenes.
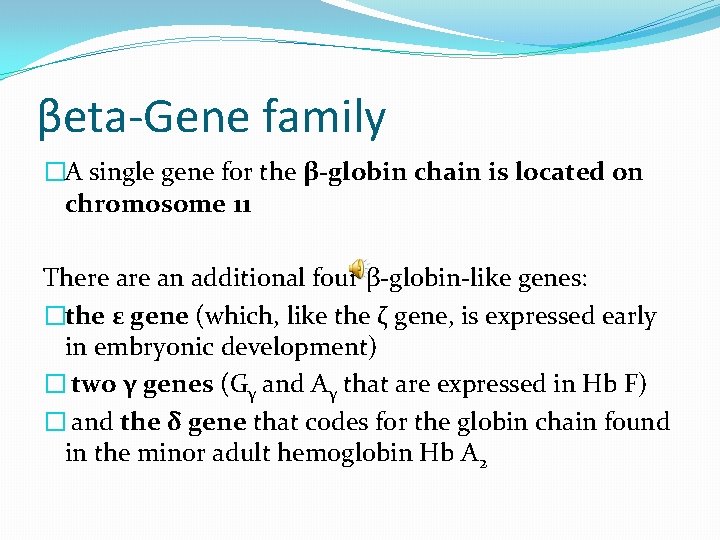
βeta-Gene family �A single gene for the β-globin chain is located on chromosome 11 There an additional four β-globin-like genes: �the ε gene (which, like the ζ gene, is expressed early in embryonic development) � two γ genes (Gγ and Aγ that are expressed in Hb F) � and the δ gene that codes for the globin chain found in the minor adult hemoglobin Hb A 2

Steps in globin chain synthesis �Expression of a globin gene begins in the nucleus of red cell precursors, where the DNA sequence encoding the gene is transcribed. �The RNA produced by transcription is actually a precursor of the messenger RNA (m. RNA) that is used as a template for the synthesis of a globin chain. �The resulting mature m. RNA enters the cytosol where its genetic information is translated producing a globin chain

- Slides: 22