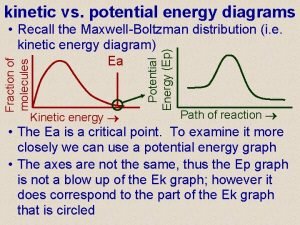
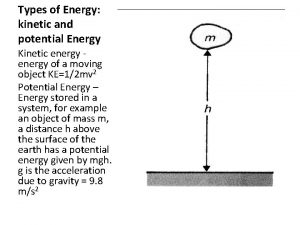
Types of Energy Gravitational Potential Spring Potential Kinetic





- Slides: 14

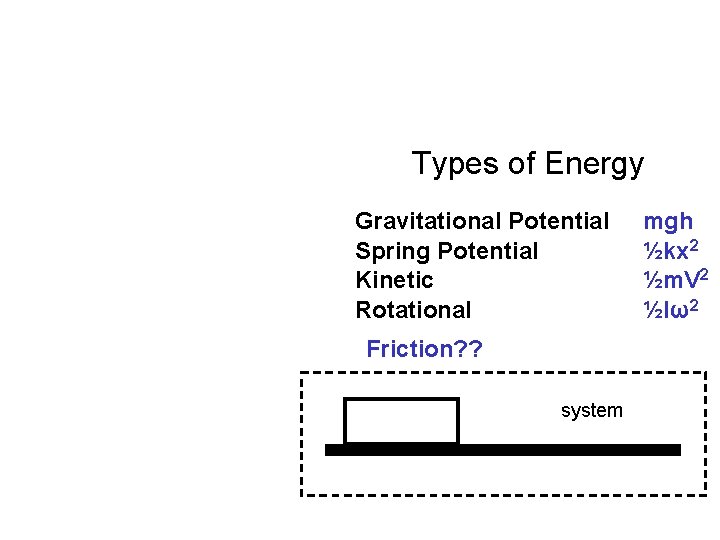
Types of Energy Gravitational Potential Spring Potential Kinetic Rotational Friction? ? system mgh ½kx 2 ½m. V 2 ½Iω2

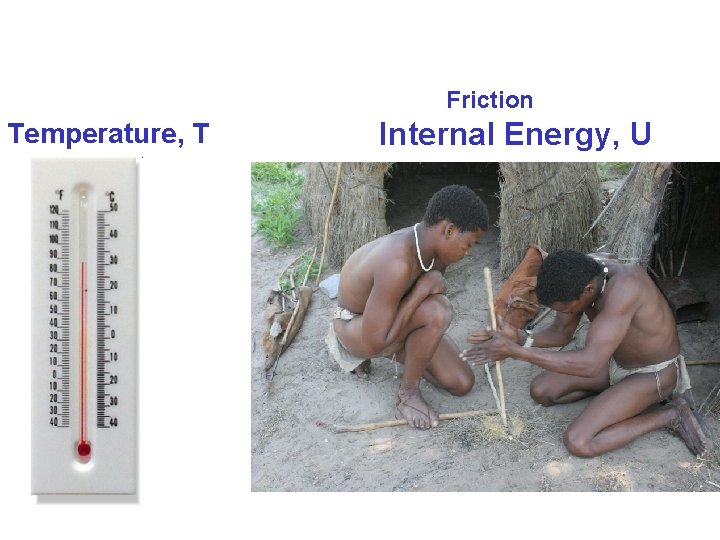
Internal Energy, U the energy associated with the microscopic components of the system It includes kinetic and potential energy of translational, rotational and vibrational motion of the atoms or molecules

Friction Temperature, T Internal Energy, U

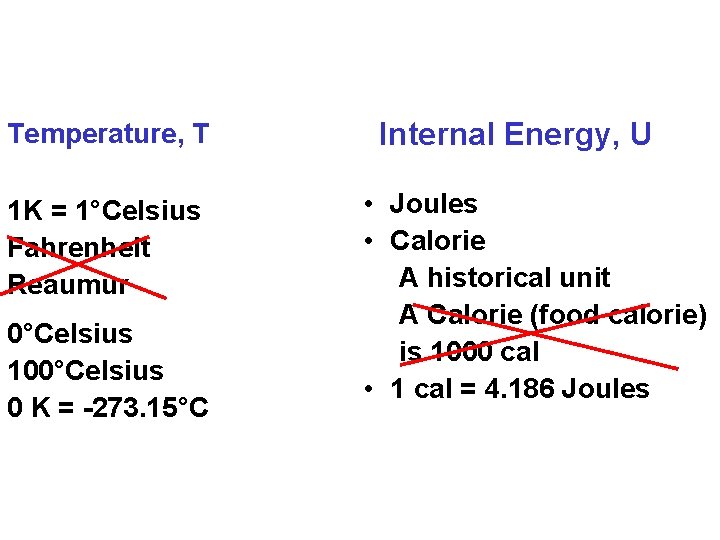
Temperature, T 1 K = 1°Celsius Fahrenheit Reaumur 0°Celsius 100°Celsius 0 K = -273. 15°C Internal Energy, U • Joules • Calorie A historical unit A Calorie (food calorie) is 1000 cal • 1 cal = 4. 186 Joules

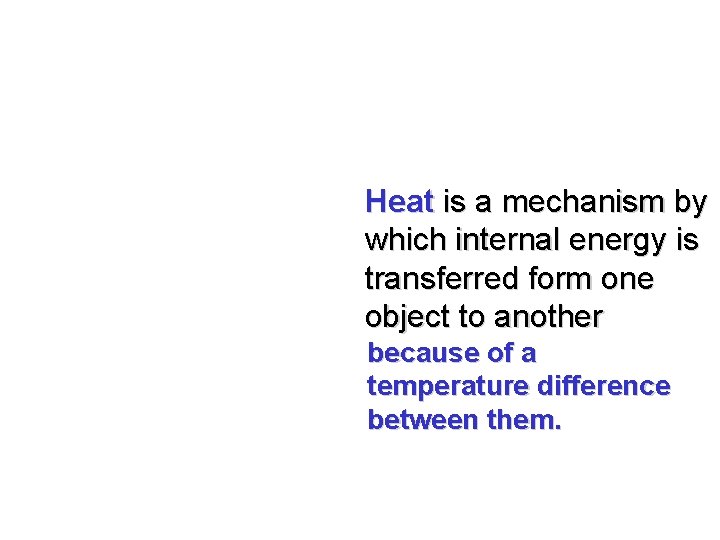
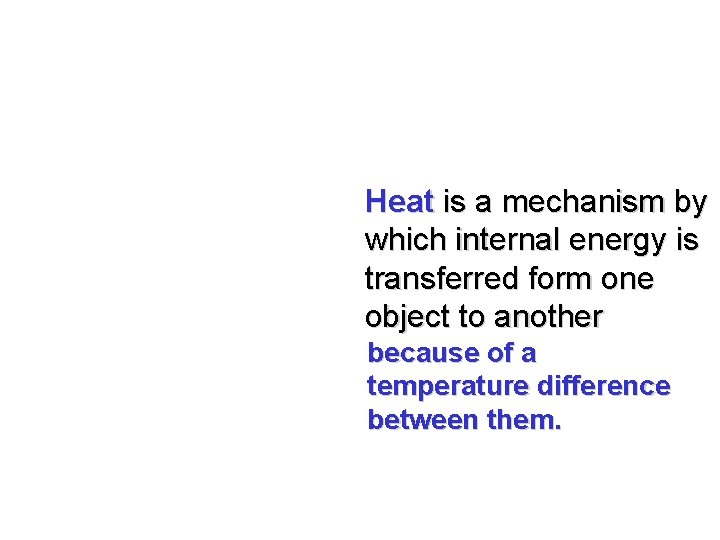
Heat is a mechanism by which internal energy is transferred form one object to another because of a temperature difference between them.

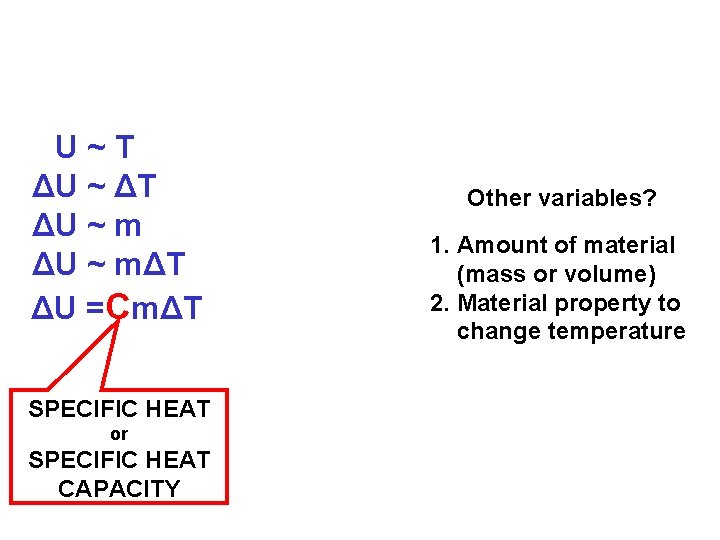
U~T ΔU ~ ΔT ΔU ~ mΔT ΔU =CmΔT SPECIFIC HEAT or SPECIFIC HEAT CAPACITY Other variables? 1. Amount of material (mass or volume) 2. Material property to change temperature

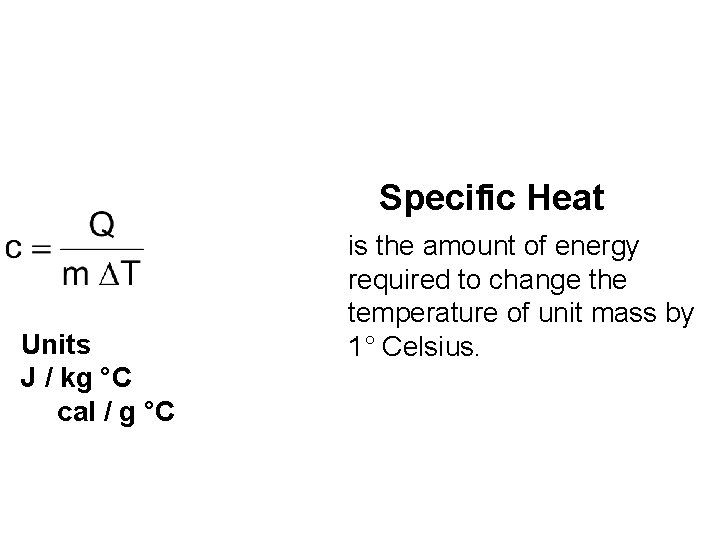
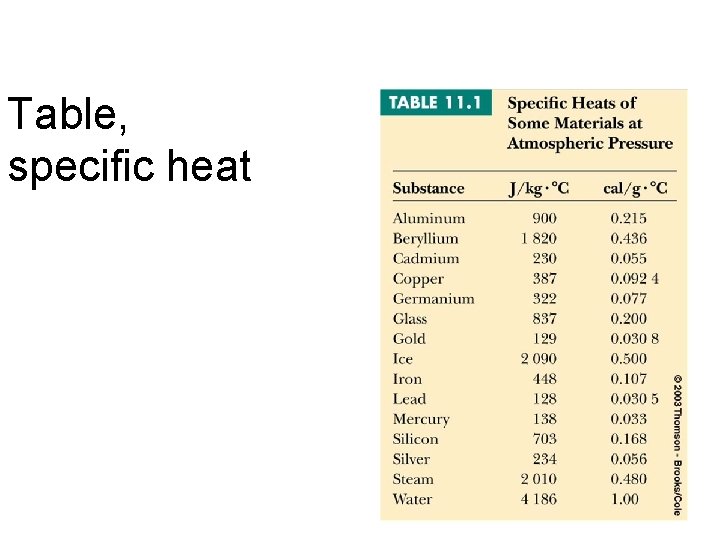
Specific Heat Units J / kg °C cal / g °C is the amount of energy required to change the temperature of unit mass by 1° Celsius.

ΔU =CmΔT Cup A Cup B The same Cup A contains 100 grams of water and cup B contains twice as much water. The water in both cups was initially at room temperature 25°C. Cup A was heated to 75°C and cup B was heated to 50°C. Which cup had more heat energy transferred to it?

A) Lower than 0°C B) 0°C C) between 0°C and 25°C D) 25°C E) Between 25°C and 50°C F) 50°C G) Higher than 50°C Cup A contains 100 grams of water at 0°C and cup B contains 100 grams of water at 50°C. The contents of the two cups are mixed together in an insulated container (no heat can transfer in or out). What is the final temperature of the water in the container?

A) Lower than 0°C B) 0°C C) between 0°C and 25°C D) 25°C E) Between 25°C and 50°C F) 50°C G) Higher than 50°C Cup A contains 100 grams of water at 0°C and cup B contains 200 grams of water at 50°C. The contents of the two cups are mixed together in an insulated container (no heat can transfer in or out). What is the final temperature of the water in the container?

Table, specific heat

Heat is a mechanism by which internal energy is transferred form one object to another because of a temperature difference between them.

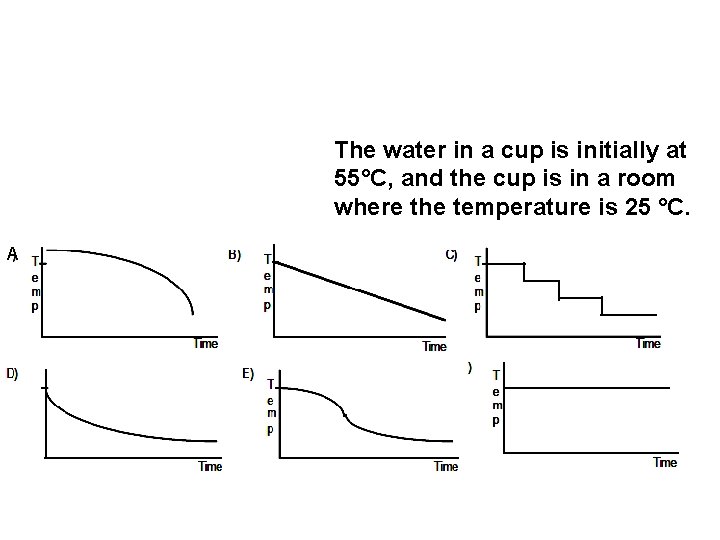
The water in a cup is initially at 55°C, and the cup is in a room where the temperature is 25 °C. A

HW. Chapter 11. 1, 11. 2, 11. 3 Quizzes and Examples!!! Problems 2, 10, 16, 24
 Gravity and kinetic energy
Gravity and kinetic energy Gravitational potential energy vs kinetic energy
Gravitational potential energy vs kinetic energy Potential energy of spring
Potential energy of spring Potential energy vs kinetic energy
Potential energy vs kinetic energy Potential energy example
Potential energy example Formula of potential energy
Formula of potential energy The change in mechanical energy
The change in mechanical energy Gravitational potential energy equation
Gravitational potential energy equation Gravitional energy
Gravitional energy Gravitational potential energy
Gravitational potential energy Work done by normal force
Work done by normal force What is potential energy
What is potential energy Examples of electrical energy
Examples of electrical energy Gpe meaning
Gpe meaning Thermal energy equation
Thermal energy equation