Types of Compounds Sec 3 2 Ionic compounds








- Slides: 11

Types of Compounds Sec. 3. 2: Ionic compounds

Ionic compounds are… Chemical compounds that consist of charged ions with opposite charges. What is an ion? Ion comes from the Greek word meaning “to wander” n Ions are ELEMENTS whose electrons have gone traveling, and when they meet up with an opposite ion, the WHOLE atom comes with!

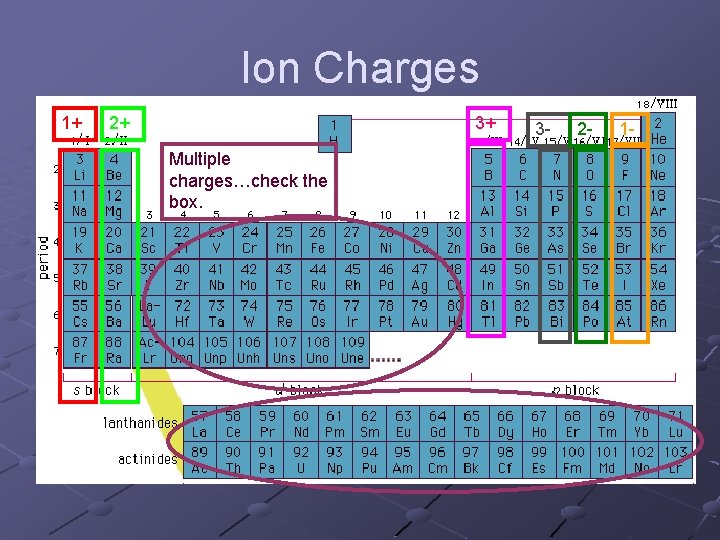
Some ions have a positive charge (+) and some have a negative charge (-). n n Metals are (+) Non-metals are (-) We use superscripts to show the ion charge. A superscript is … A tiny letter/ number above the term, like an exponent. Ex. Na+, Sn 2+, O 2 -, F-

Ion Charges 1+ 2+ 3+ Multiple charges…check the box. 3 - 2 - 1 -

In order to write an ionic formula, you need to know the ion charge. This is because ionic compounds are always neutral… n there are the same numbers of positives as negatives Ex: Water. n n Ion = H+ and O 2 -. There needs to be 2 H+ so the charges can equal out. H+, H+ , O 2 We write this as H 20(l)

There are three simple steps to follow: Turn to page 147 and paraphrase each step in the boxes below.

Step 1 Write each ion with its charge *Remember: Certain columns on the periodic table are always the same, and some have more than one choice.

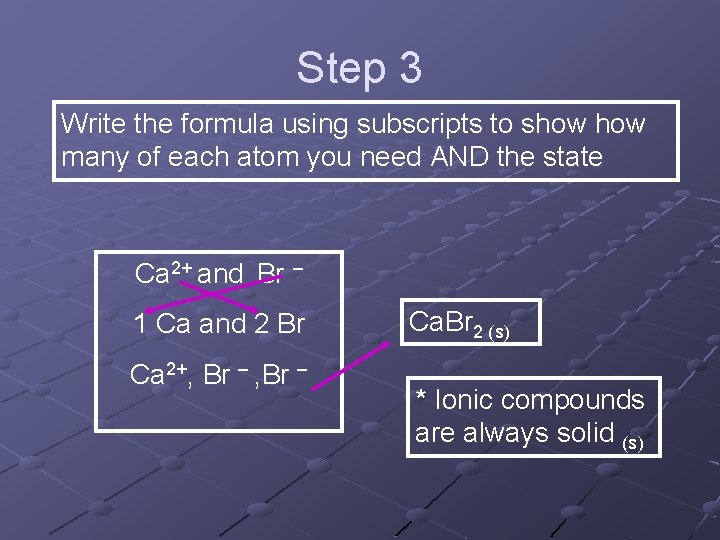
Step 2 Figure out how many of each you need to make the charges balance. The best way is to do a switch-er-oo between the number of atoms and the opposite ion’s charge. Ca 2+ and Br – 1 Ca and 2 Br Ca 2+, Br –

Step 3 Write the formula using subscripts to show many of each atom you need AND the state Ca 2+ and Br – 1 Ca and 2 Br Ca 2+, Br – Ca. Br 2 (s) * Ionic compounds are always solid (s)

Multiple charges… These are called polyatomic ions. n Poly means “multiple” or “more than one”. In order to know which of the ion charges you need the formula will have a roman numeral in it. Cr 2+ is written chromium (II) Co 3+ is written cobalt (III)

Do C & R Questions 1 -8 on page 149