Types of Chemical Rxns Composition Synthesis Rxns Two









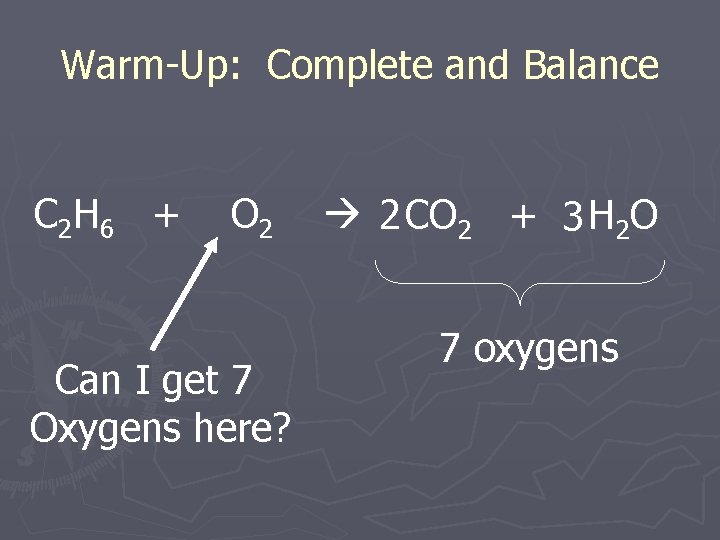
- Slides: 30

Types of Chemical Rxns

Composition (Synthesis) Rxns Two or more substances react to form a single, more complex substance. Examples: 2 Hg + O 2 2 Hg. O 2 Ag + S Ag 2 S Movie of Potassium + Bromine

Decomposition Reactions A Complex Substance is Broken Down (usually by heating) into 2 or more smaller substances endo ____thermic Examples Mn. O 2 KCl. O 3 Side + heat 2 Which does energy term+ 3 go 2 KCl O 2 on? ªH Ba. Cl 2 2 H 2 O Mn. O 2 2 H 2 O 2 (aq) Ba. Cl 2 + 2 H 2 O 2 H 2 O (l) + O 2 (g)+ heat Movie of Nitrogen Tri. Iodide

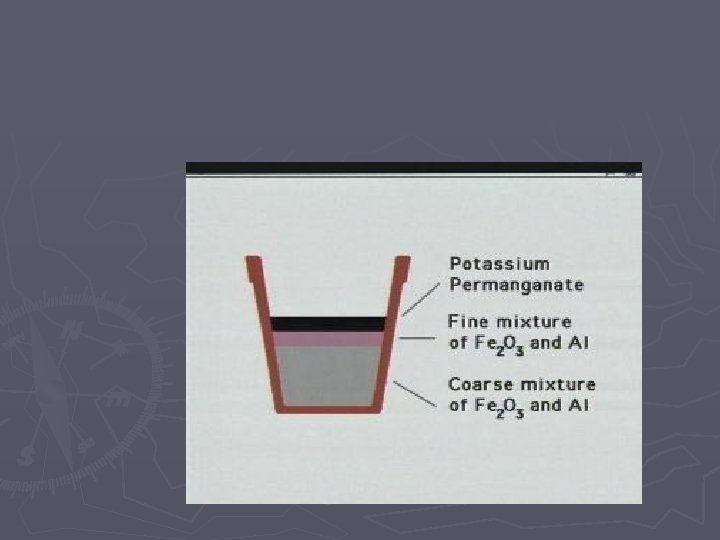
Single Replacement Rxns A single element takes the place of another element in a compound Examples: Zn + 2 HCl Cu + 2 Ag. NO 3 H 2 + Zn. Cl 2 2 Ag + Cu(NO 3)2 Movie of Thermite Reaction


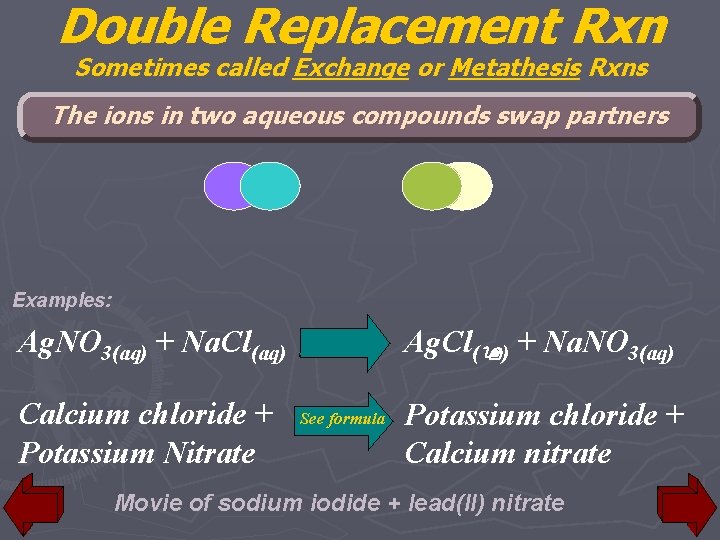
Double Replacement Rxn Sometimes called Exchange or Metathesis Rxns The ions in two aqueous compounds swap partners Examples: Ag. Cl(9) + Na. NO 3(aq) Ag. NO 3(aq) + Na. Cl(aq) Calcium chloride + Potassium Nitrate See formula Potassium chloride + Calcium nitrate Movie of sodium iodide + lead(II) nitrate

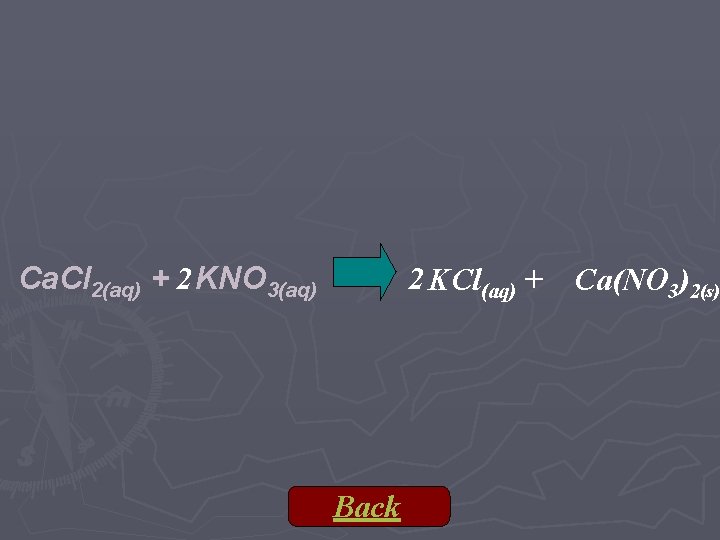
Ca. Cl 2(aq) + 2 KNO 3(aq) 2 KCl(aq) + Back Ca(NO 3)2(s)

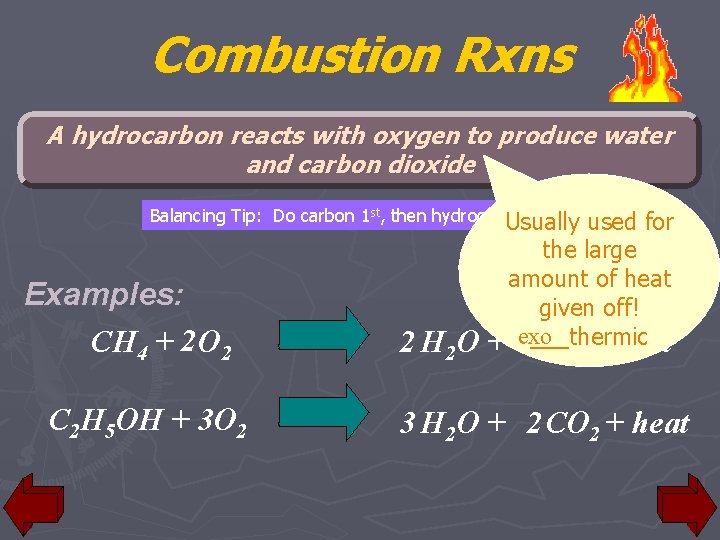
Combustion Rxns A hydrocarbon reacts with oxygen to produce water and carbon dioxide Balancing Tip: Do carbon 1 st, then hydrogen, Usually then oxygen used Examples: CH 4 + 2 O 2 C 2 H 5 OH + 3 O 2 2 H 2 O for the large amount of heat given off! ___thermic + exo CO + heat 2 3 H 2 O + 2 CO 2 + heat

Combustion Rxns Most reactions that we think of as ‘burning’ a fuel are combustion reactions Examples: • Burning a log of Wood to heat your s’mores • Burning Coal to heat water to create electricity at a power plant • Grilling with Propane to heat your hot dog Hydrocarbon • Burning Gasoline in your car to heat the air and push pistons • Burning Oil Hydrocarbon in your furnace to heat your house Hydrocarbon

Combustion Rxns Some downsides to combustion reactions: ► Fossil Fuels are non-renewable resources § We are using them much faster than they are being produced § Eventually, we will run out of fossil fuels

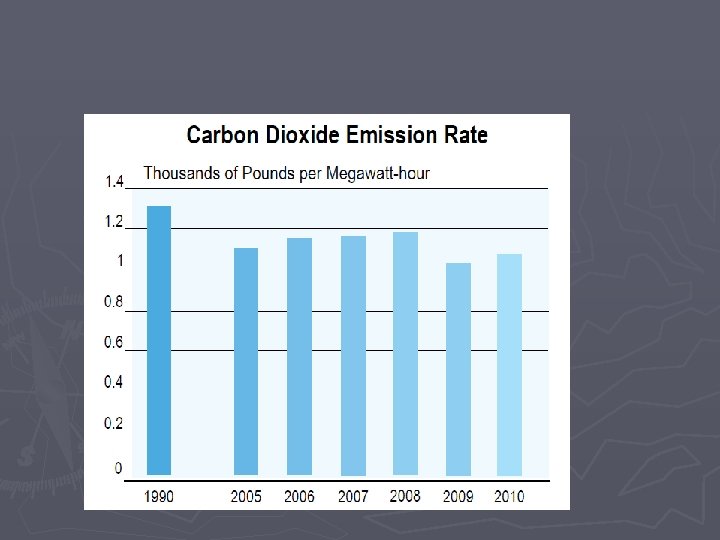
Combustion Rxns Some downsides to combustion reactions: CO 2 is a greenhouse gas and increasing CO 2 levels contributes to global warming – (others are methane, water vapor, ozone, nitrous oxide) Our widespread burning of fossil fuels over the last hundred years has increased the earth’s CO 2 levels

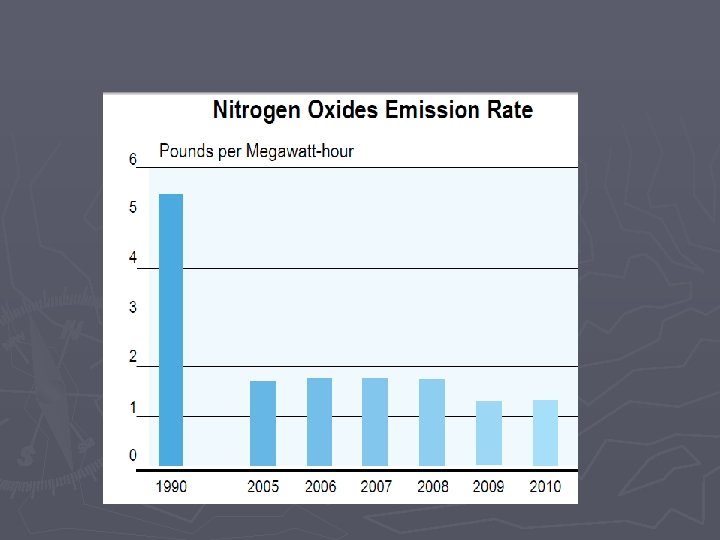
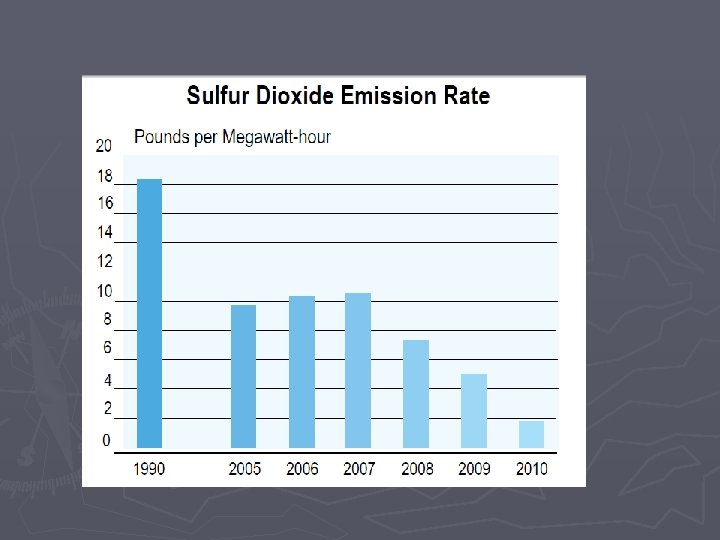
Combustion Rxns Some downsides to combustion reactions: ► Combustion will be “incomplete” when not enough oxygen is present § Complete Combustion ►Hydrocarbon + O 2 H 2 O + CO 2 § Incomplete combustion results in CO, Carbon (soot), and nitrous oxides ►Hydrocarbon + O 2 C + CO +H 2 O + CO 2 ►CO is poisonous ►Nitrous oxides are bad for the environment (acid rain, smog)

Combustion Rxns What can be done? ► Incomplete combustion can be improved by better designed burners / engines ► Catalytic converters in vehicle reduce CO ► “Scrubbers” placed in industrial exhaust systems can reduce nitrous oxides and CO None of these “fixes” cuts down or removes CO 2 emissions Balancing Combustion




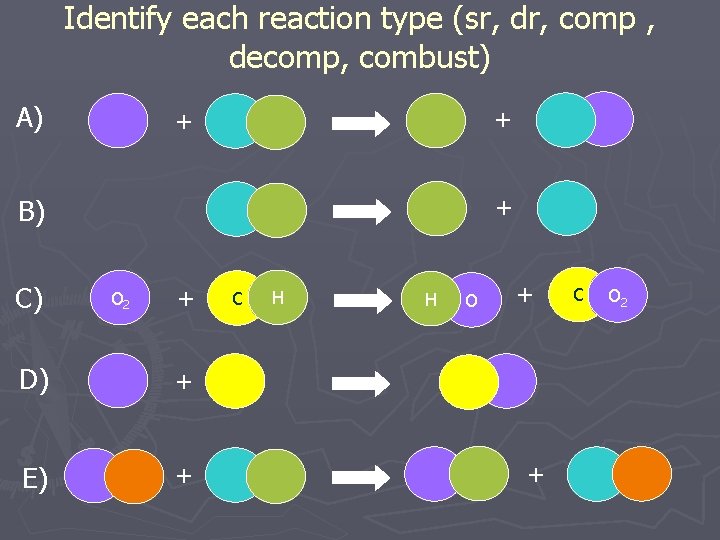
Identify each reaction type (sr, dr, comp , decomp, combust) A) + + + B) C) O 2 + D) + E) + C H H O + + C O 2

A Closer Look: Composition Rxns ► Aluminum + oxygen ► Calcium oxide + water ► Sulfur + oxygen

More Composition Rxns ► Sn + O 2 -----> ► Sodium + chlorine----> ► SO 3 + H 2 O ----> ► Lithium oxide + water--> ► ► ► Silicon + oxygen

More Composition Rxns Answers ► Sn ► ► + O 2 ----->Sn. O 2 2 Na + Cl 2 ---> 2 Na. Cl SO 3 + H 2 O ----> H 2 SO 4 Li 2 O + H 2 O ------> 2 Li. OH Si + O 2 -----> Si. O 2

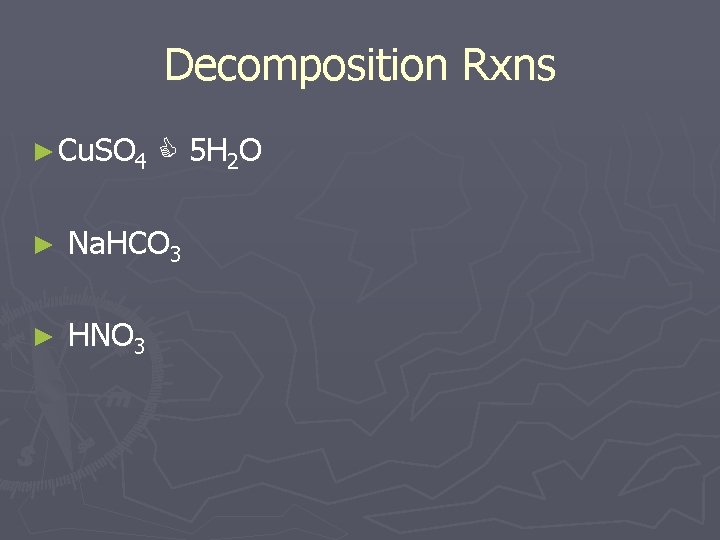
Decomposition Rxns ► Cu. SO 4 5 H 2 O ► Na. HCO 3 ► HNO 3

More Decomposition Rxns ► Na 2 CO 3 ----> ► Mg. O -----> ► Mg. SO 4 7 H 2 O ----> ► Sr(NO 3)2 ----> ► H 2 CO 3 ---->

More Decomposition Rxns (Answers) ► Na 2 CO 3 ► ► ----> Na 2 O + CO 2 2 Mg. O -----> 2 Mg + O 2 Mg. SO 4*7 H 2 O ----> Mg. SO 4 + 7 H 2 O Sr(NO 3)2 ----> Sr(NO 2)2 + O 2 H 2 CO 3 ----> H 2 O + CO 2
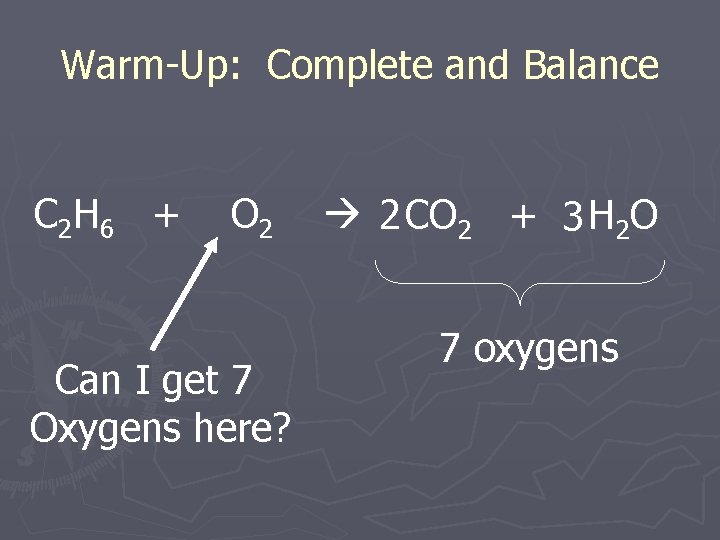
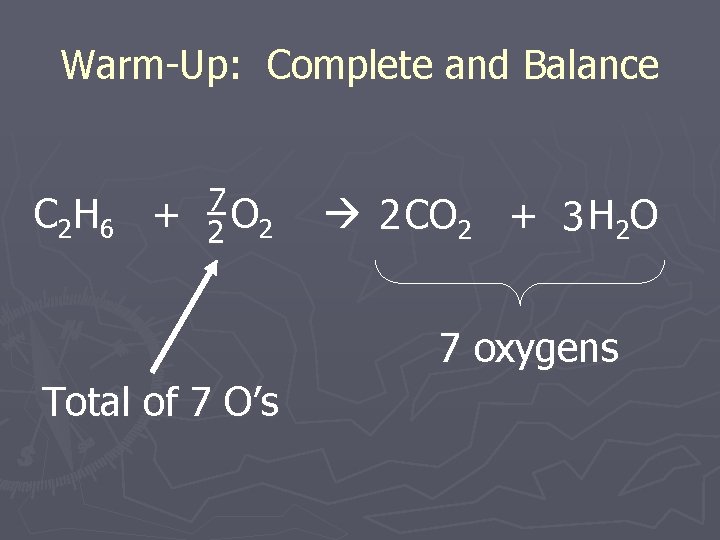
Warm-Up: Complete and Balance C 2 H 6 + O 2 Can I get 7 Oxygens here? 2 CO 2 + 3 H 2 O 7 oxygens

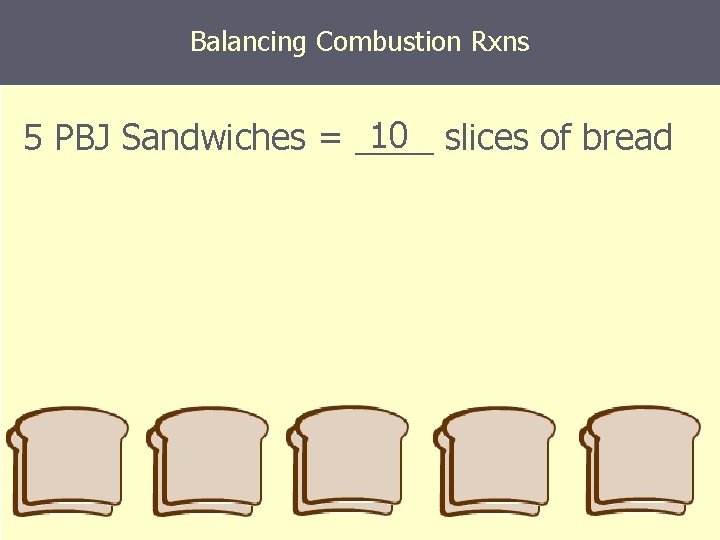
Balancing Combustion Rxns 10 slices of bread 5 PBJ Sandwiches = ____

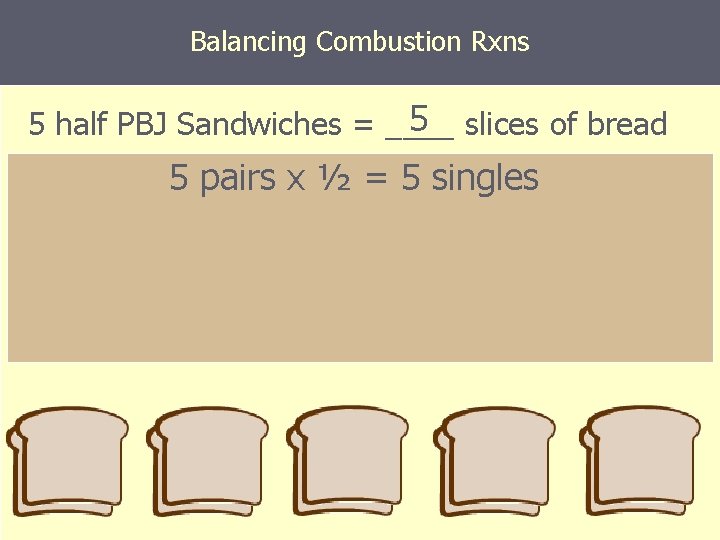
Balancing Combustion Rxns 5 slices of bread 5 half PBJ Sandwiches = ____ 5 pairs x ½ = 5 singles

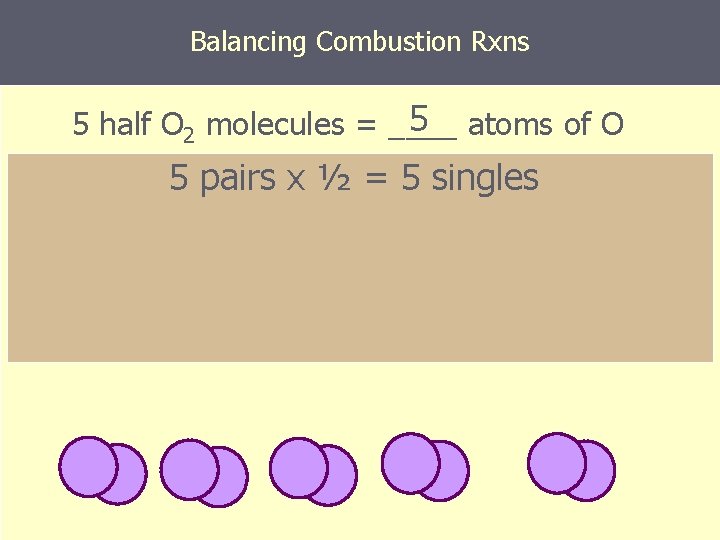
Balancing Combustion Rxns 5 atoms of O 5 half O 2 molecules = ____ 5 pairs x ½ = 5 singles

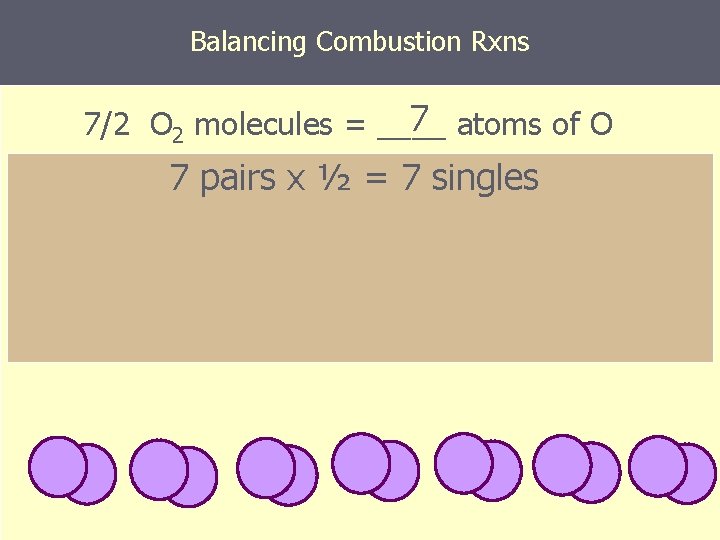
Balancing Combustion Rxns 7 atoms of O 7/2 O 2 molecules = ____ 7 pairs x ½ = 7 singles

Warm-Up: Complete and Balance C 2 H 6 + 7 2 O 2 2 CO 2 + 3 H 2 O 7 oxygens Total of 7 O’s

Your Turn: ► Complete packet # 10 -12 on Page 14 in your