TYPES OF CHEMICAL RXNS COMBUSTION SO FAR We
TYPES OF CHEMICAL RXNS: COMBUSTION

SO FAR We have learned 4 types of rxns: X + Y XY Decomposition: XY X + Y Single Displacement: X + YZ XZ + Y Double Displacement: XW + YZ XZ + YW Today we learn about Combustion Synthesis:

COMBUSTION This rxn involves a fuel burning or reacting quickly with oxygen. Oxygen is ALWAYS a reactant in combustion! Without it, the rxn won’t happen. The products of the rxn are usually an Oxide and energy. We typically use Hydrocarbons (compounds made up of Carbon & Hydrogen) as the fuel Ex. C 3 H 8 is known as propane (BBQ) and C 4 H 10 is called butane and is used for cooking or lighters.

COMPLETE COMBUSTION OF HYDROCARBONS In general, if a lot of Oxygen is available, then Hydrocarbons can be burnt completely. If we have complete combustion, the only products available are CO 2, H 2 O, and Energy Complete combustion usually produces a bluish flame The general rxn for Complete Combustion of Hydrocarbons is, CXHY + O 2 CO 2 + H 2 O + Energy If we don’t have complete combustion, you end up with a sooty residue (carbon), like in a fireplace.

EXAMPLE The complete combustion of Ethane in air is described by the following chemical eqn. C 2 H 6(g) + O 2 CO 2(g) + H 2 O(g) + Energy (Skeleton Eqn. ) Balance the above equation. 2 C 2 H 6(g) + 7 O 2 4 CO 2(g) + 6 H 2 O(g) + Energy (Balanced Eqn. )
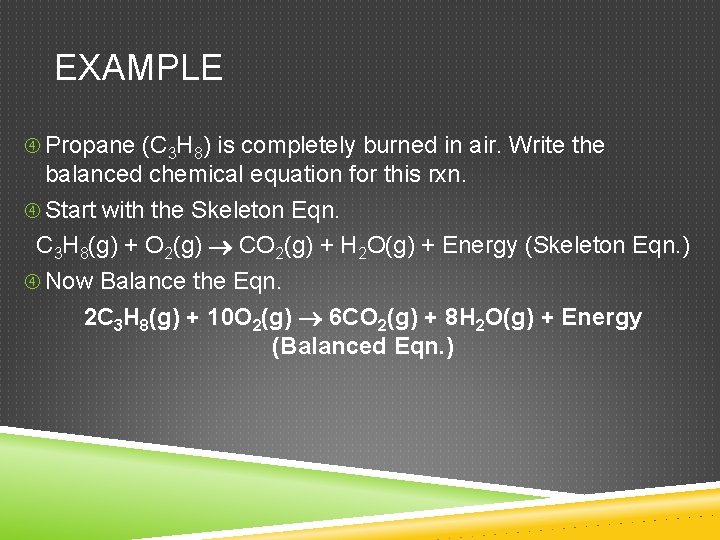
EXAMPLE Propane (C 3 H 8) is completely burned in air. Write the balanced chemical equation for this rxn. Start with the Skeleton Eqn. C 3 H 8(g) + O 2(g) CO 2(g) + H 2 O(g) + Energy (Skeleton Eqn. ) Now Balance the Eqn. 2 C 3 H 8(g) + 10 O 2(g) 6 CO 2(g) + 8 H 2 O(g) + Energy (Balanced Eqn. )
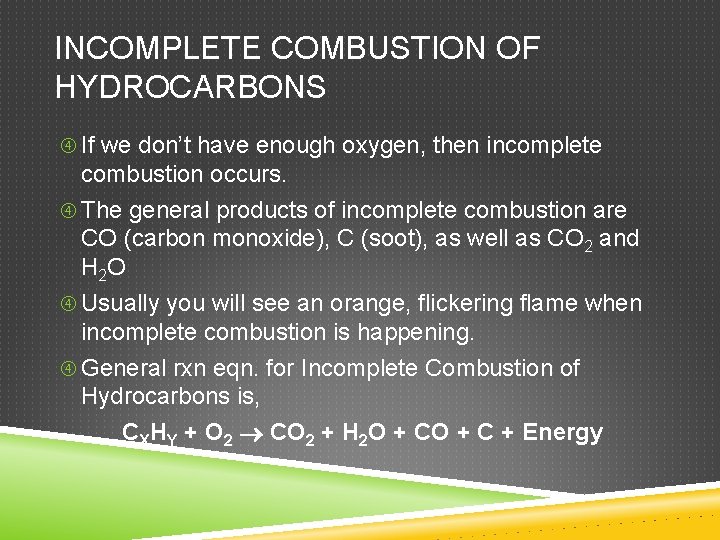
INCOMPLETE COMBUSTION OF HYDROCARBONS If we don’t have enough oxygen, then incomplete combustion occurs. The general products of incomplete combustion are CO (carbon monoxide), C (soot), as well as CO 2 and H 2 O Usually you will see an orange, flickering flame when incomplete combustion is happening. General rxn eqn. for Incomplete Combustion of Hydrocarbons is, CXHY + O 2 CO 2 + H 2 O + C + Energy
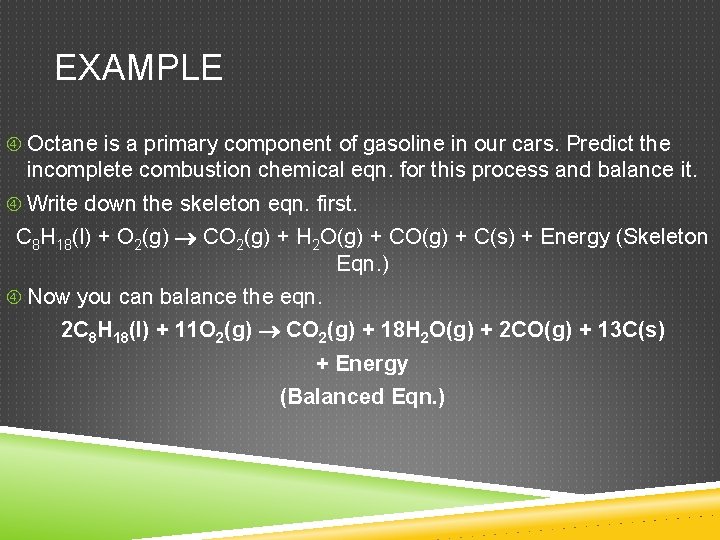
EXAMPLE Octane is a primary component of gasoline in our cars. Predict the incomplete combustion chemical eqn. for this process and balance it. Write down the skeleton eqn. first. C 8 H 18(l) + O 2(g) CO 2(g) + H 2 O(g) + C(s) + Energy (Skeleton Eqn. ) Now you can balance the eqn. 2 C 8 H 18(l) + 11 O 2(g) CO 2(g) + 18 H 2 O(g) + 2 CO(g) + 13 C(s) + Energy (Balanced Eqn. )

WHAT IF IT ISN’T A HYDROCARBON? We can burn other things, like metals in the presence of Oxygen. The general eqn. for combustion of other substances is, Element + Oxygen Oxide X + O 2 XO If you haven’t noticed, Combustion of other elements is also a Synthesis rxn!

EXAMPLE Write the word equation for burning sodium in air. Also create the balanced eqn. associated with this rxn. Start by writing the word eqn. Sodium + Oxygen Sodium Oxide Next, write the skeleton eqn. Na(s) + O 2(g) Na 2 O(s) (Skeleton Eqn. ) Finally, balance the eqn. 4 Na(s) + O 2(g) 2 Na 2 O(s) (Balanced Eqn. )

HOMEWORK Read Section 6. 9 Pg. 251, #2 – 5
- Slides: 11