Types of Chemical Reactions Vocabulary Coefficient In a
Types of Chemical Reactions
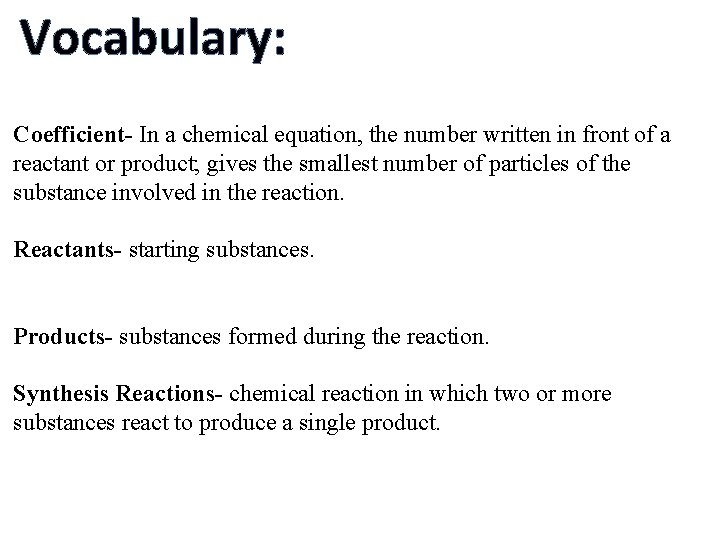
Vocabulary: Coefficient- In a chemical equation, the number written in front of a reactant or product; gives the smallest number of particles of the substance involved in the reaction. Reactants- starting substances. Products- substances formed during the reaction. Synthesis Reactions- chemical reaction in which two or more substances react to produce a single product.
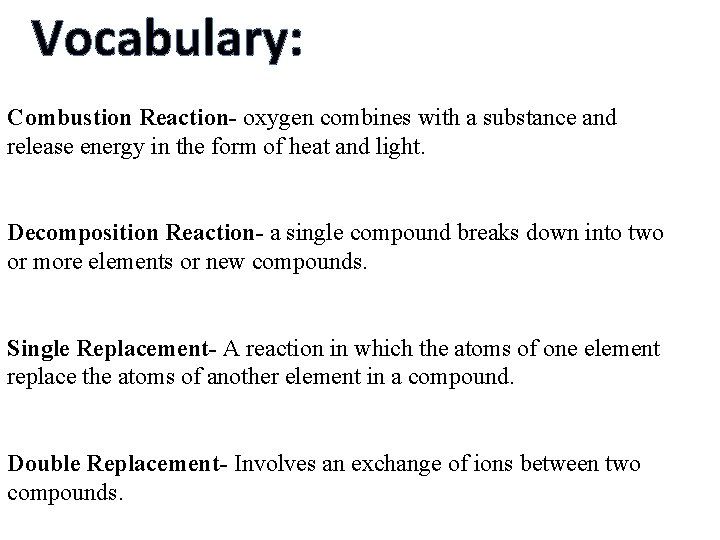
Vocabulary: Combustion Reaction- oxygen combines with a substance and release energy in the form of heat and light. Decomposition Reaction- a single compound breaks down into two or more elements or new compounds. Single Replacement- A reaction in which the atoms of one element replace the atoms of another element in a compound. Double Replacement- Involves an exchange of ions between two compounds.
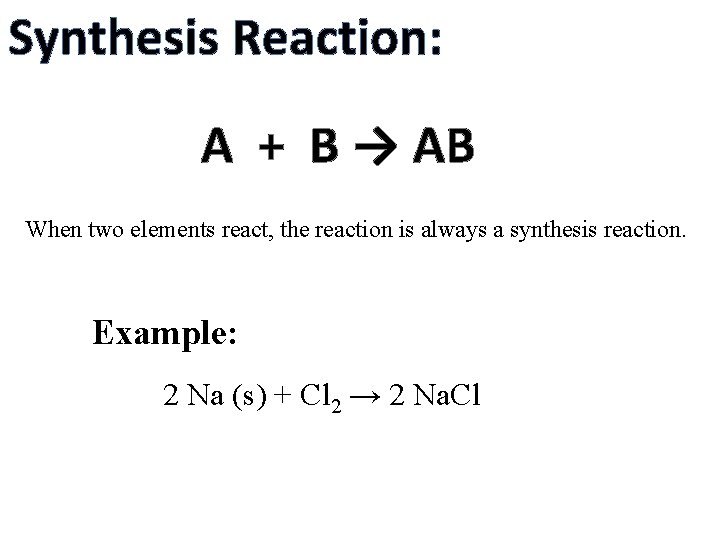
Synthesis Reaction: A + B → AB When two elements react, the reaction is always a synthesis reaction. Example: 2 Na (s) + Cl 2 → 2 Na. Cl
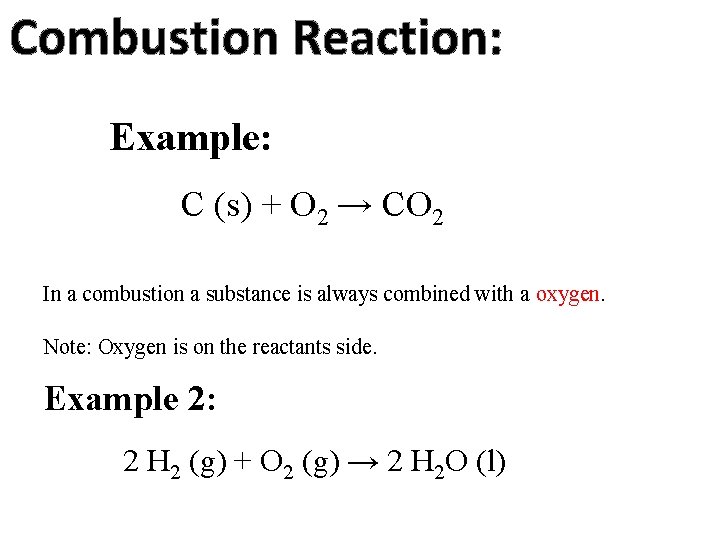
Combustion Reaction: Example: C (s) + O 2 → CO 2 In a combustion a substance is always combined with a oxygen. Note: Oxygen is on the reactants side. Example 2: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l)
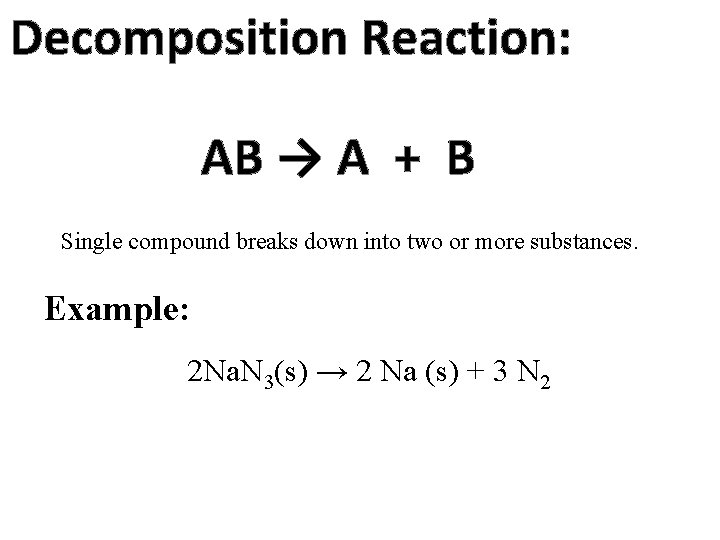
Decomposition Reaction: AB → A + B Single compound breaks down into two or more substances. Example: 2 Na. N 3(s) → 2 Na (s) + 3 N 2
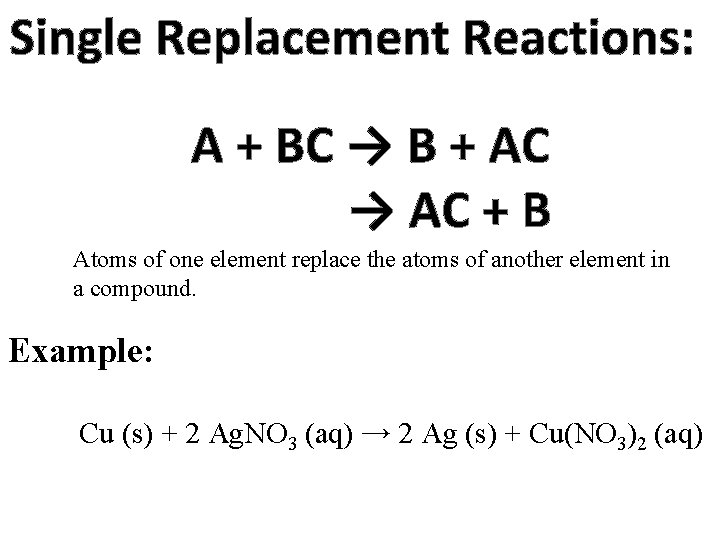
Single Replacement Reactions: A + BC → B + AC → AC + B Atoms of one element replace the atoms of another element in a compound. Example: Cu (s) + 2 Ag. NO 3 (aq) → 2 Ag (s) + Cu(NO 3)2 (aq)
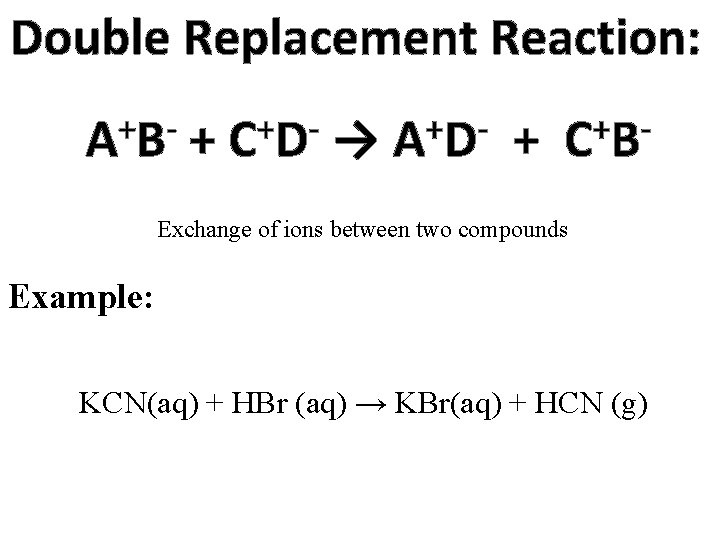
Double Replacement Reaction: + AB + + CD → + AD + + CB Exchange of ions between two compounds Example: KCN(aq) + HBr (aq) → KBr(aq) + HCN (g)
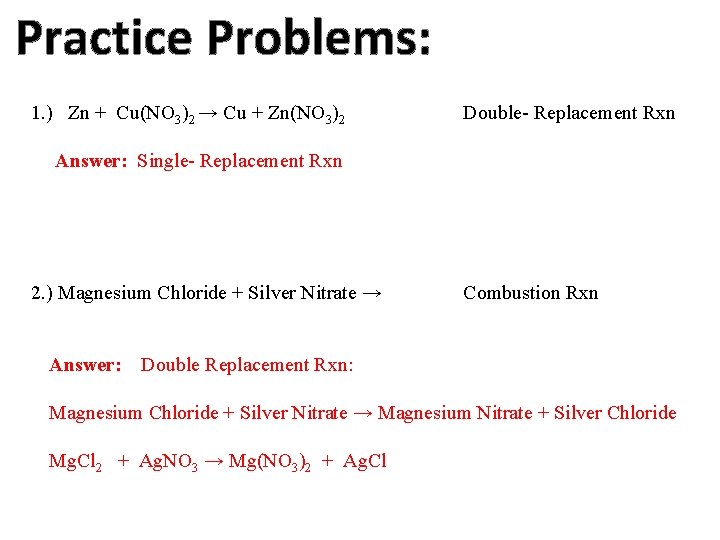
Practice Problems: 1. ) Zn + Cu(NO 3)2 → Cu + Zn(NO 3)2 Double- Replacement Rxn Answer: Single- Replacement Rxn 2. ) Magnesium Chloride + Silver Nitrate → Answer: Combustion Rxn Double Replacement Rxn: Magnesium Chloride + Silver Nitrate → Magnesium Nitrate + Silver Chloride Mg. Cl 2 + Ag. NO 3 → Mg(NO 3)2 + Ag. Cl
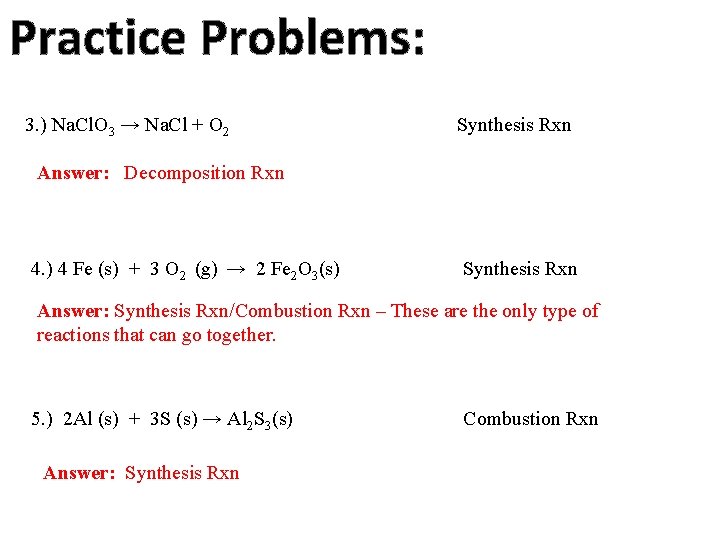
Practice Problems: 3. ) Na. Cl. O 3 → Na. Cl + O 2 Synthesis Rxn Answer: Decomposition Rxn 4. ) 4 Fe (s) + 3 O 2 (g) → 2 Fe 2 O 3(s) Synthesis Rxn Answer: Synthesis Rxn/Combustion Rxn – These are the only type of reactions that can go together. 5. ) 2 Al (s) + 3 S (s) → Al 2 S 3(s) Answer: Synthesis Rxn Combustion Rxn
- Slides: 10