TYPES OF CHEMICAL REACTIONS There are five types









- Slides: 27

TYPES OF CHEMICAL REACTIONS

There are five types of chemical reactions you need to know: 1. 2. 3. 4. 5. Synthesis reactions Decomposition reactions Single displacement reactions Double displacement reactions Combustion reactions

By the end of this lesson you should be able to: ü Classify the type of reaction ü Predict the product(s) of the reaction ü Balance the final equation including state symbols

Some steps for doing reactions 1. Identify the type of reaction 2. Predict the product(s) using the type of reaction as a model 3. Balance it § Don’t forget about the diatomic elements! § (Br I N Cl H O F) For example, Oxygen is O 2 which is a compound

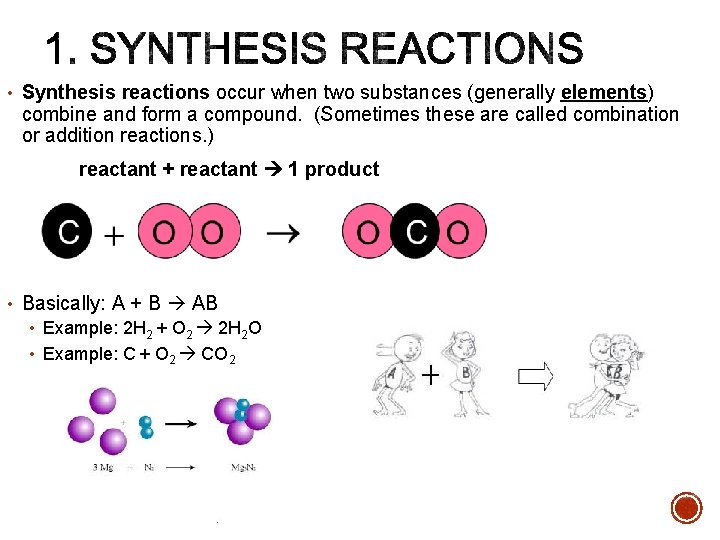
• Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions. ) reactant + reactant 1 product • Basically: A + B AB • Example: 2 H 2 + O 2 2 H 2 O • Example: C + O 2 CO 2

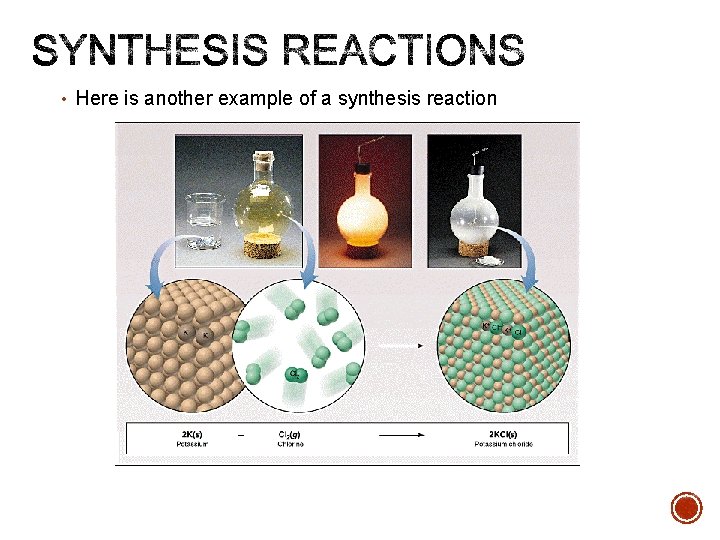
• Here is another example of a synthesis reaction

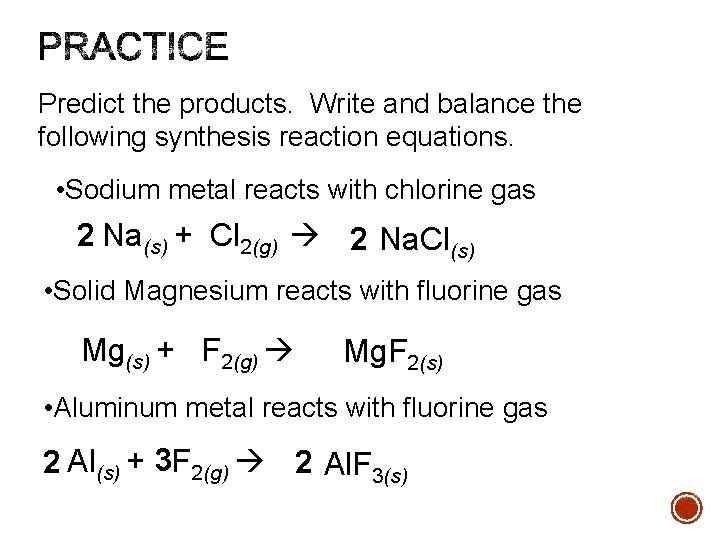
Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas 2 Na(s) + Cl 2(g) 2 Na. Cl(s) • Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) Mg. F 2(s) • Aluminum metal reacts with fluorine gas 2 Al(s) + 3 F 2(g) 2 Al. F 3(s)

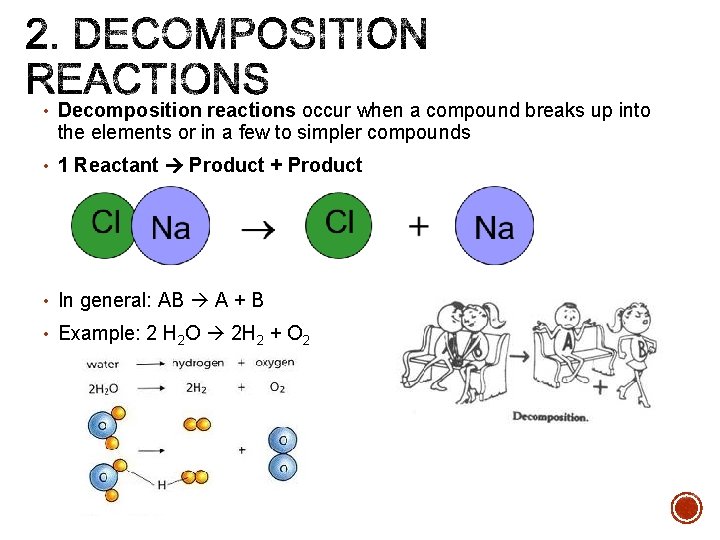
• Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds • 1 Reactant Product + Product • In general: AB A + B • Example: 2 H 2 O 2 H 2 + O 2

• Another view of a decomposition reaction:

• Carbonates and chlorates are special case decomposition reactions that do not go to the elements. • Carbonates (CO 32 -) decompose to carbon dioxide and a metal oxide • Example: Ca. CO 3 CO 2 + Ca. O • Chlorates (Cl. O 3 -) decompose to oxygen gas and a metal chloride • Example: 2 Al(Cl. O 3)3 2 Al. Cl 3 + 9 O 2 • There are other special cases, but we will not explore those in this year

Predict the products. Then, write and balance the following decomposition reaction equations: • Solid Lead (IV) oxide decomposes Pb. O 2(s) • Aluminum nitride decomposes Al. N(s)

Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: N 2(g) + O 2(g) Nitrogen monoxide Ba. CO 3(s) Co(s)+ S(s) (make Co be +3) NH 3(g) + H 2 CO 3(aq) NI 3(s)

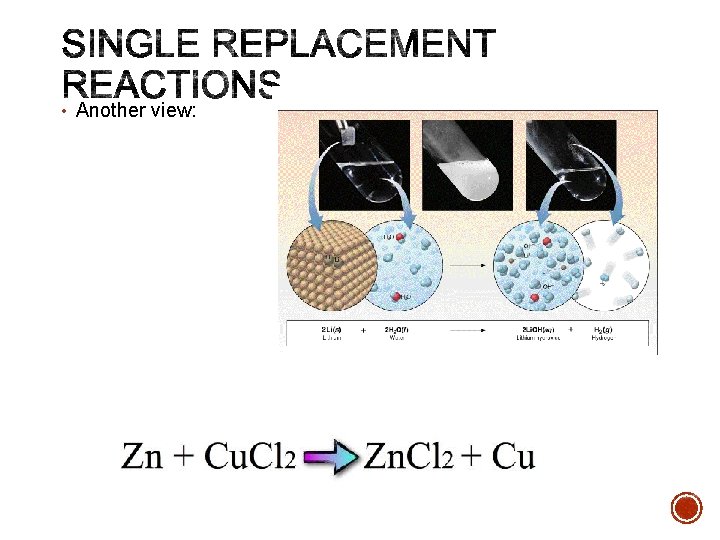
• Single Replacement Reactions occur when one element replaces another in a compound. • A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compound A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (remember the cation always goes first!) When H 20 splits into ions, it splits into H+ and OH-

• Another view:

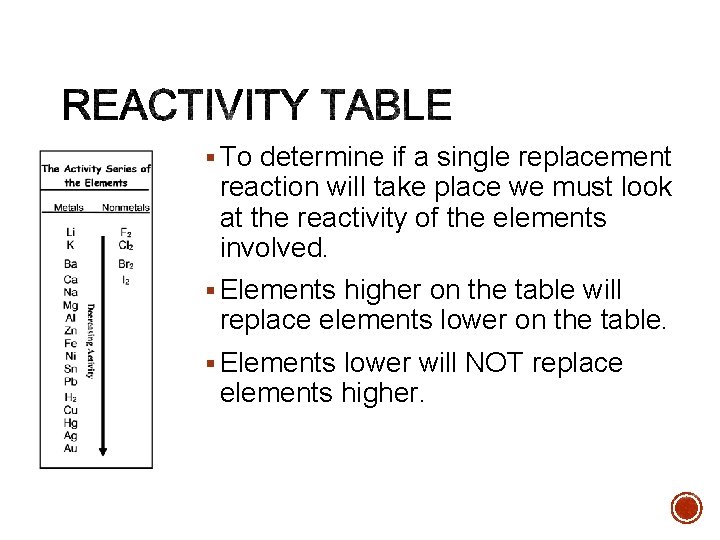
§ To determine if a single replacement reaction will take place we must look at the reactivity of the elements involved. § Elements higher on the table will replace elements lower on the table. § Elements lower will NOT replace elements higher.

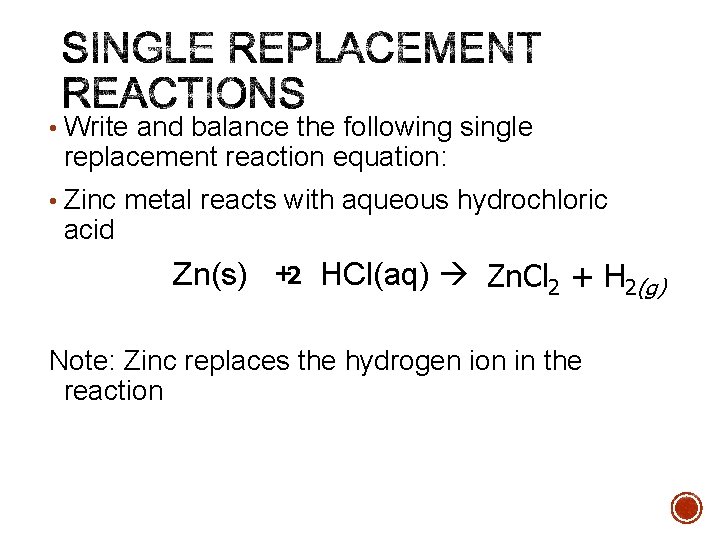
• Write and balance the following single replacement reaction equation: • Zinc metal reacts with aqueous hydrochloric acid Zn(s) +2 HCl(aq) Zn. Cl 2 + H 2(g) Note: Zinc replaces the hydrogen ion in the reaction

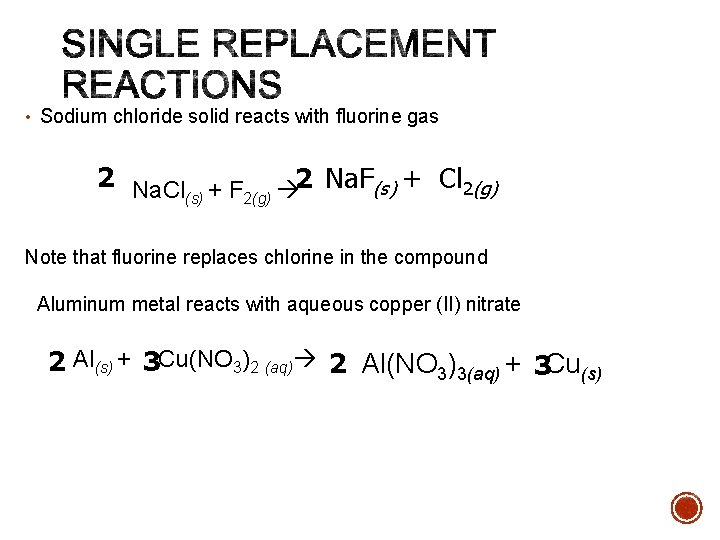
• Sodium chloride solid reacts with fluorine gas 2 Na. Cl + F 2 Na. F(s) + Cl 2(g) (s) 2(g) Note that fluorine replaces chlorine in the compound Aluminum metal reacts with aqueous copper (II) nitrate 2 Al(s) + 3 Cu(NO 3)2 (aq) 2 Al(NO 3)3(aq) + 3 Cu(s)

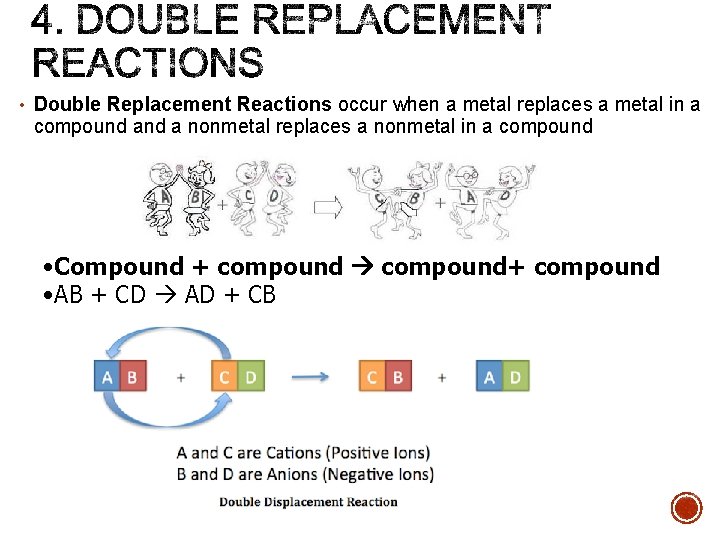
• Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound • Compound + compound+ compound • AB + CD AD + CB

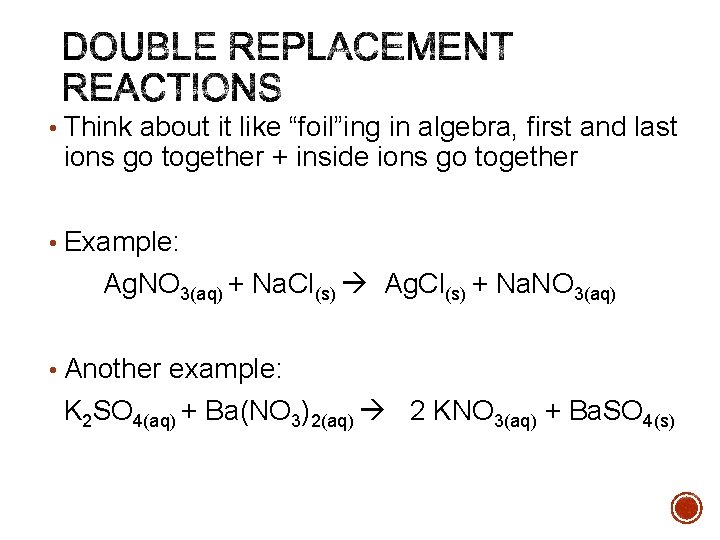
• Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together • Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) • Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) 2 KNO 3(aq) + Ba. SO 4(s)

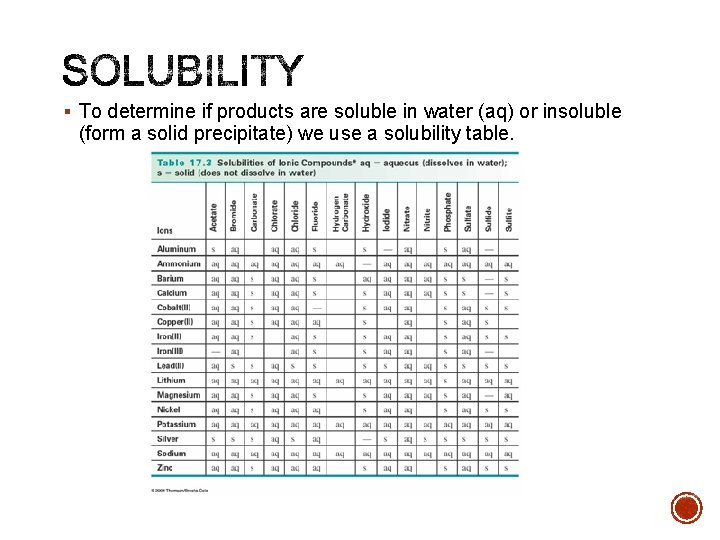
§ To determine if products are soluble in water (aq) or insoluble (form a solid precipitate) we use a solubility table.

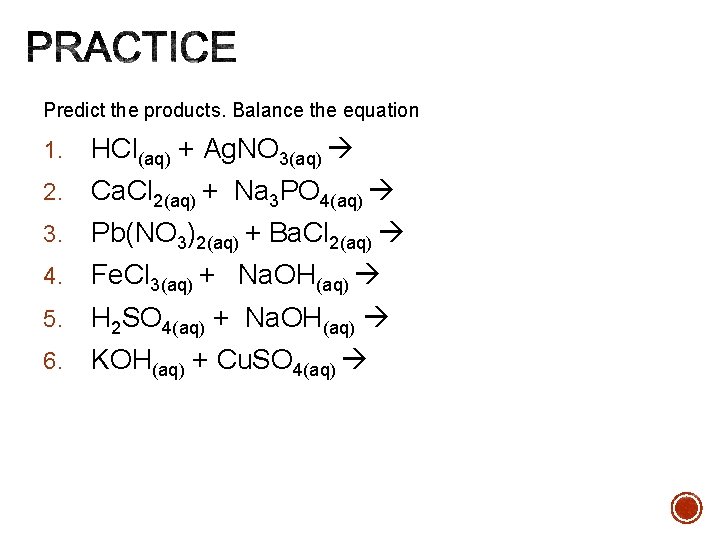
Predict the products. Balance the equation 1. HCl(aq) + Ag. NO 3(aq) 2. Ca. Cl 2(aq) + Na 3 PO 4(aq) 3. Pb(NO 3)2(aq) + Ba. Cl 2(aq) 4. Fe. Cl 3(aq) + Na. OH(aq) 5. H 2 SO 4(aq) + Na. OH(aq) 6. KOH(aq) + Cu. SO 4(aq)

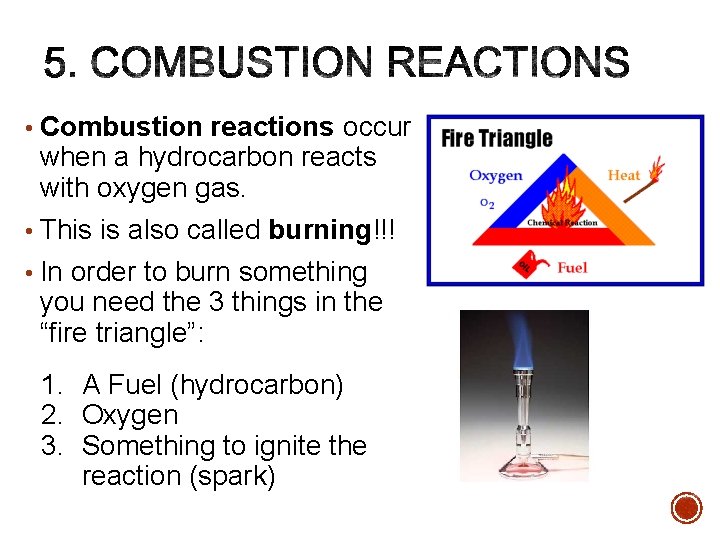
• Combustion reactions occur when a hydrocarbon reacts with oxygen gas. • This is also called burning!!! • In order to burn something you need the 3 things in the “fire triangle”: 1. A Fuel (hydrocarbon) 2. Oxygen 3. Something to ignite the reaction (spark)

• In general: • Cx. Hy + O 2 CO 2 + H 2 O • Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some byproducts like carbon monoxide) • Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18)

Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.

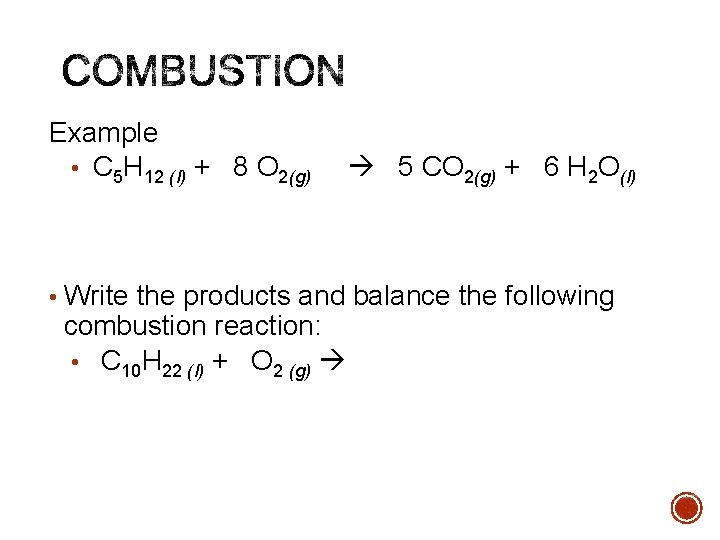
Example • C 5 H 12 (l) + 8 O 2(g) 5 CO 2(g) + 6 H 2 O(l) • Write the products and balance the following combustion reaction: • C 10 H 22 (l) + O 2 (g)

State the type of reaction, predict the products, and balance the following reactions: 1. Ba. Cl 2 + H 2 SO 4 2. C 6 H 12 + O 2 3. Zn + Cu. SO 4 4. Cs + Br 2 5. Fe. CO 3

§ https: //www. youtube. com/watch? v=g-bi. Rw. AVTV 8 Good Overall Review: § https: //www. youtube. com/watch? v=d 58 Uc. B_Yb 2 Q
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Mikael ferm
Mikael ferm Combustion chemical reaction
Combustion chemical reaction Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Five types of reactions
Five types of reactions Types of chemical reactions redox
Types of chemical reactions redox Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry How to identify type of reaction
How to identify type of reaction 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Combustion chemical reaction definition
Combustion chemical reaction definition 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Examples of double replacement reactions
Examples of double replacement reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Three types of chemical reactions
Three types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Redox examples
Redox examples Unit 5 chemical reactions answers
Unit 5 chemical reactions answers There are five types of
There are five types of Stoichiometry island diagram
Stoichiometry island diagram Unit 5 chemical reactions
Unit 5 chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions