Types of chemical reactions The 5 reactions Combustion

Types of chemical reactions The 5 reactions
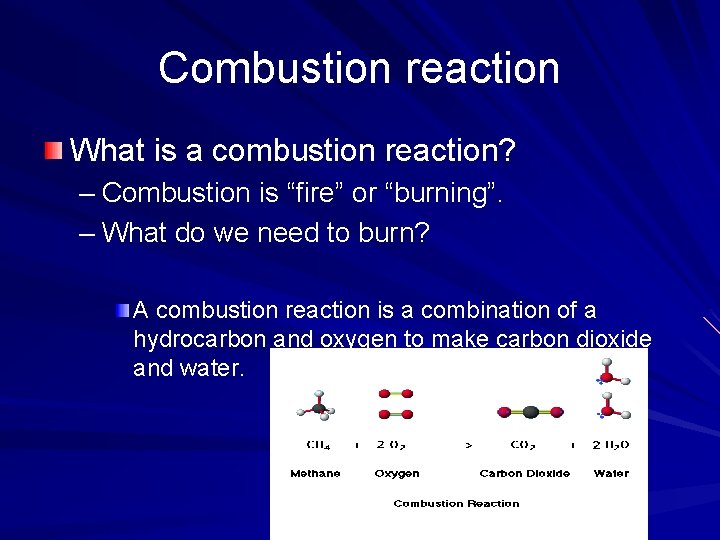
Combustion reaction What is a combustion reaction? – Combustion is “fire” or “burning”. – What do we need to burn? A combustion reaction is a combination of a hydrocarbon and oxygen to make carbon dioxide and water.

Synthesis reaction What is a synthesis reaction? – Synthetic is “man made”. – Synthesis means to make. – What does this mean in a reaction? A synthesis reaction is a combination of 2 or more reactants to make one product. – H 2 + O 2 H 2 O

Decomposition reaction What is a decomposition reaction? – Decompose means to break down. – How does this relate to a reaction? A decomposition reaction is when one reactant breaks down into multiple products. – H 2 O H 2 + O 2

Replacement reaction What is a replacement reaction? – Replace means to take the place of. – What does this mean for a reaction? Replacement reactions occur when one substance takes the place of another in a compound.
Single replacement What is a single replacement reaction? – One thing is being replaced. A single replacement reaction is when one chemical takes the place of a chemical already in a compound. AB + Y AY + B AB + Y YB + A
Double replacement What is a double replacement reaction? – This is where 2 chemicals in different compounds are replaced. A double displacement reaction is when 2 compounds switch partners. AB + XY AY + XB AB + XY AX + BY

- Slides: 8