Types of Chemical Reactions T Trimpe http sciencespot
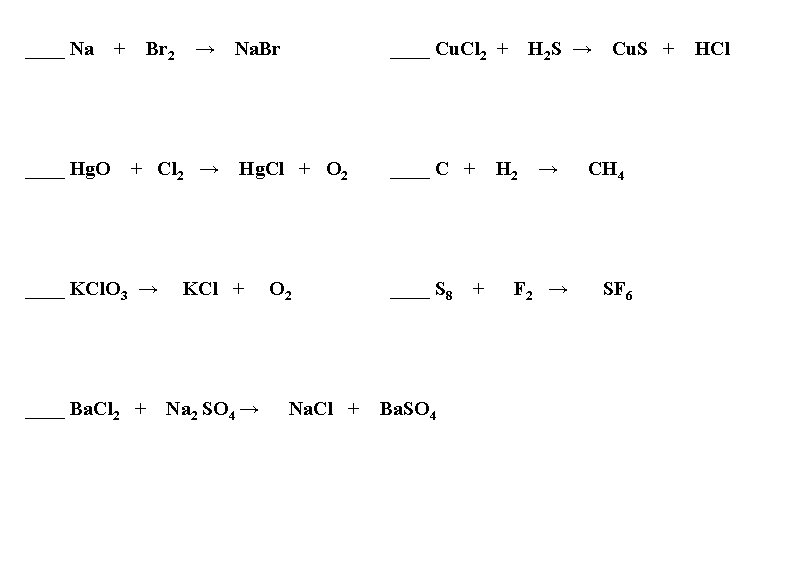

- Slides: 9

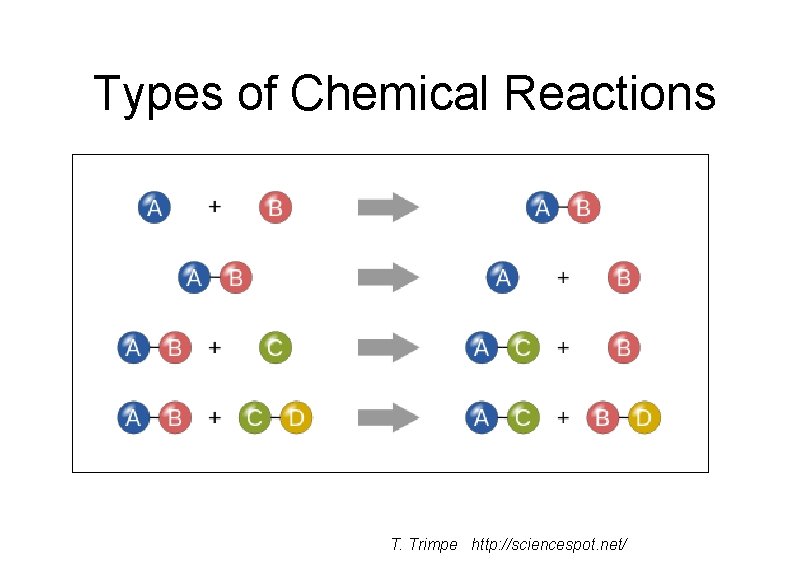
Types of Chemical Reactions T. Trimpe http: //sciencespot. net/

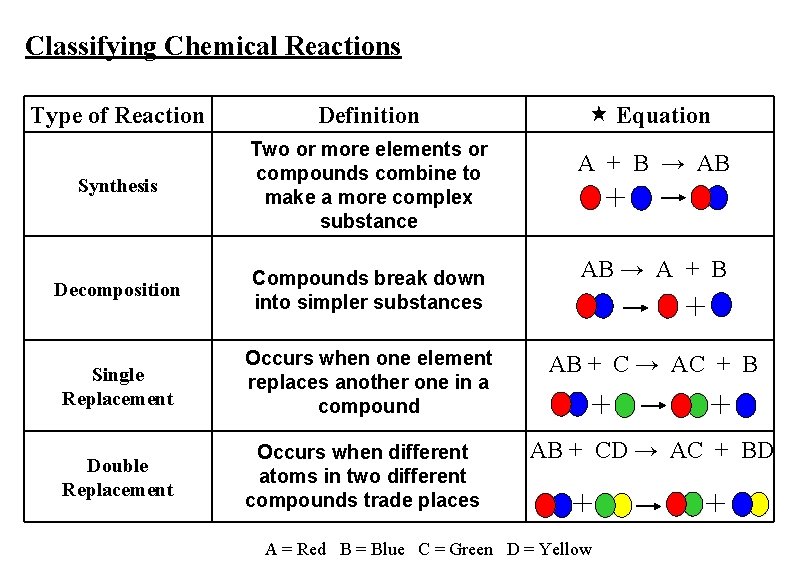
Classifying Chemical Reactions Equation Type of Reaction Definition Synthesis Two or more elements or compounds combine to make a more complex substance Decomposition Compounds break down into simpler substances Single Replacement Occurs when one element replaces another one in a compound AB + C → AC + B Double Replacement Occurs when different atoms in two different compounds trade places AB + CD → AC + BD A + B → AB AB → A + B A = Red B = Blue C = Green D = Yellow

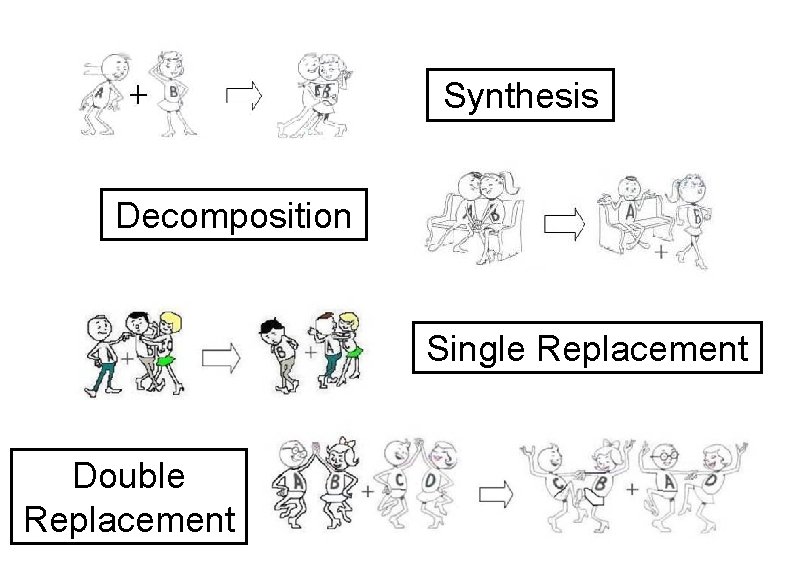
Synthesis Decomposition Single Replacement Double Replacement

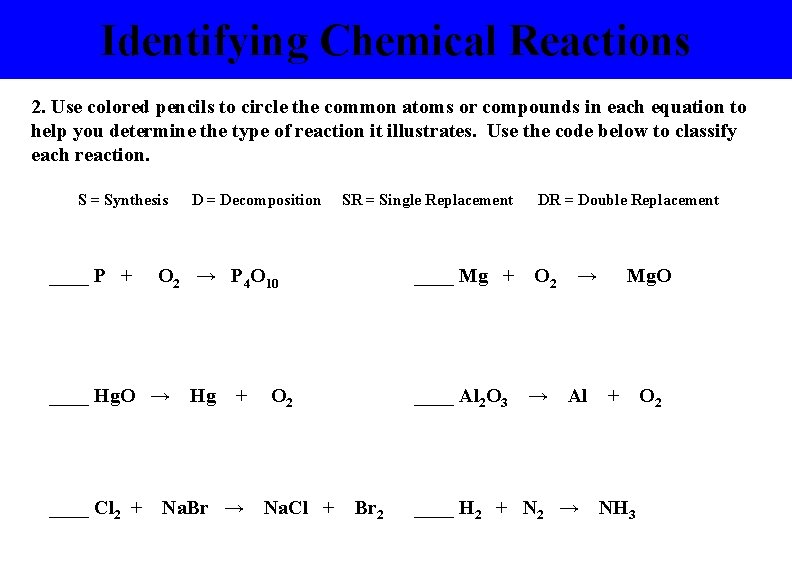
Identifying Chemical Reactions 2. Use colored pencils to circle the common atoms or compounds in each equation to help you determine the type of reaction it illustrates. Use the code below to classify each reaction. S = Synthesis ____ P + SR = Single Replacement O 2 → P 4 O 10 ____ Hg. O → ____ Cl 2 + D = Decomposition Hg + Na. Br → ____ Mg + O 2 Na. Cl + ____ Al 2 O 3 Br 2 DR = Double Replacement O 2 → → Al ____ H 2 + N 2 → Mg. O + NH 3 O 2
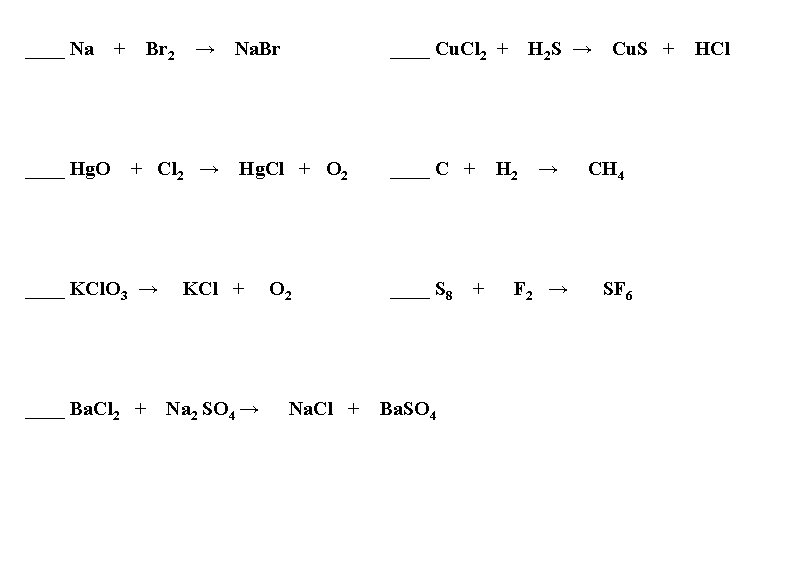
____ Na ____ Hg. O + Br 2 → + Cl 2 → Na. Br ____ Cu. Cl 2 + Hg. Cl + O 2 ____ C + ____ KCl. O 3 → KCl + ____ Ba. Cl 2 + Na 2 SO 4 → O 2 Na. Cl + ____ S 8 Ba. SO 4 + H 2 S → H 2 → F 2 → Cu. S + CH 4 SF 6 HCl

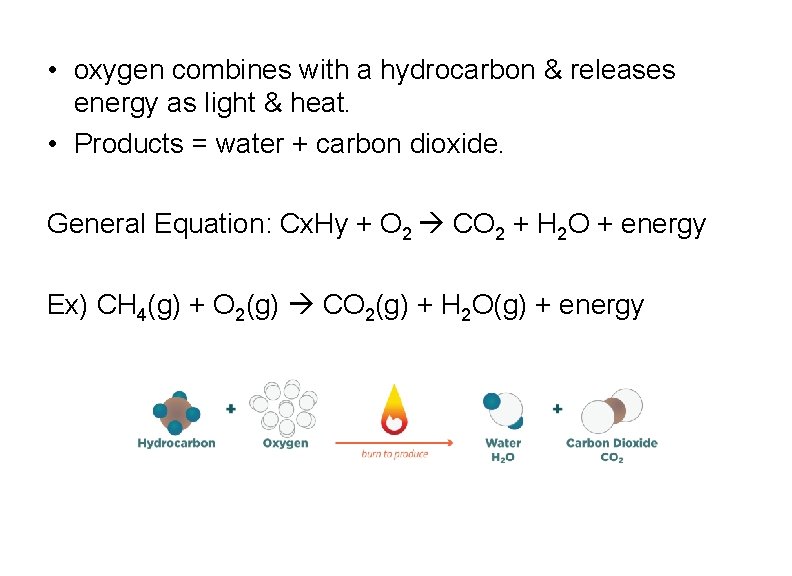
A Couple Special Cases 1. Combustion

• oxygen combines with a hydrocarbon & releases energy as light & heat. • Products = water + carbon dioxide. General Equation: Cx. Hy + O 2 CO 2 + H 2 O + energy Ex) CH 4(g) + O 2(g) CO 2(g) + H 2 O(g) + energy

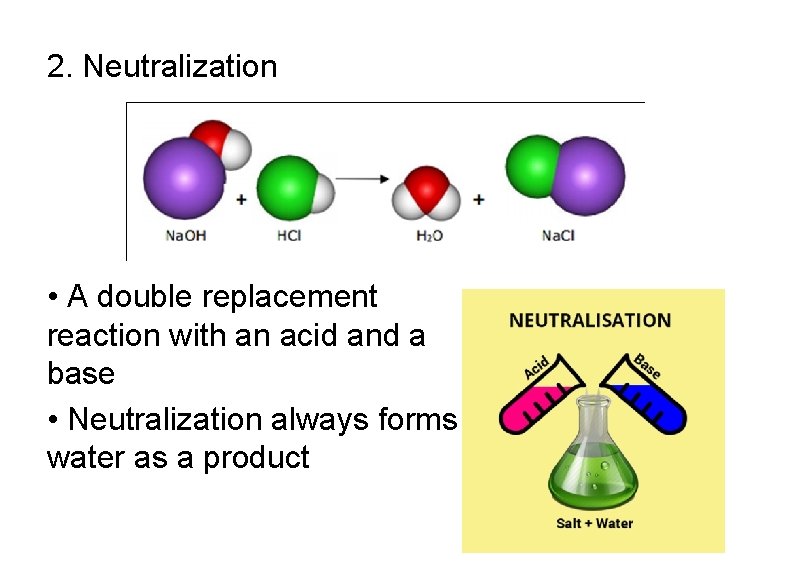
2. Neutralization • A double replacement reaction with an acid and a base • Neutralization always forms water as a product

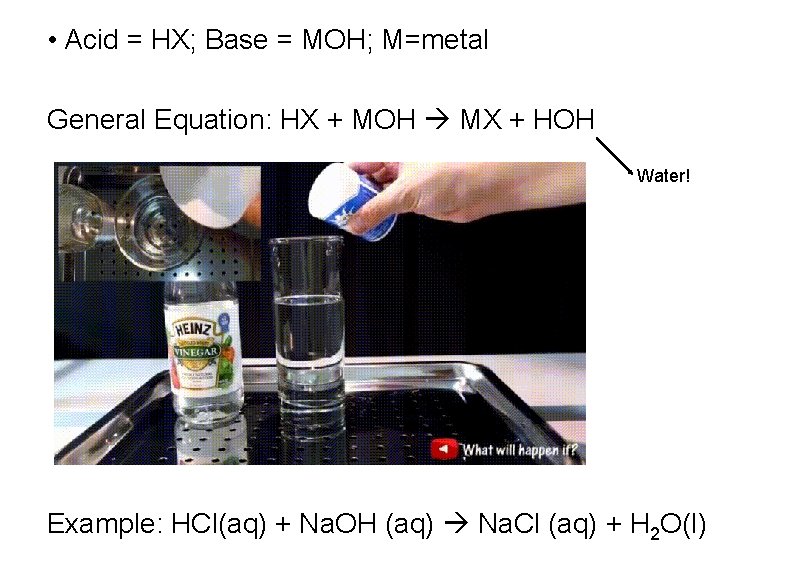
• Acid = HX; Base = MOH; M=metal General Equation: HX + MOH MX + HOH Water! Example: HCl(aq) + Na. OH (aq) Na. Cl (aq) + H 2 O(l)