Types of Chemical Reactions Synthesis Reactions Reactants Products



















- Slides: 27

Types of Chemical Reactions

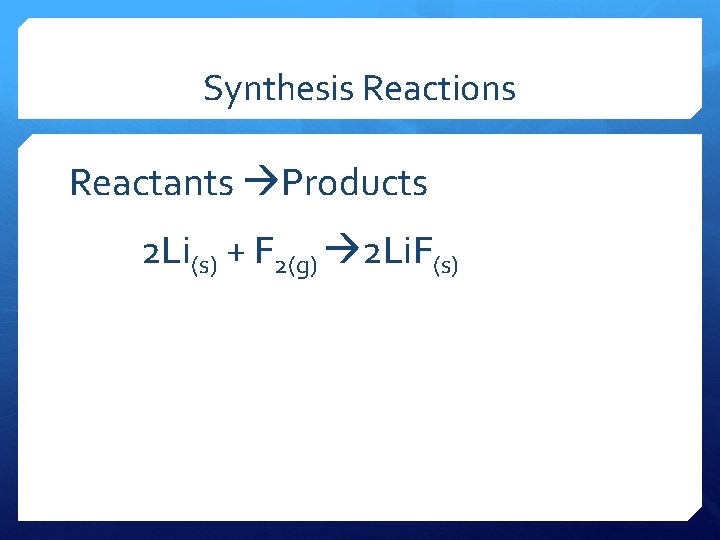
Synthesis Reactions Reactants Products 2 Li(s) + F 2(g) 2 Li. F(s)

Na. Cl Crystal

Synthesis Reaction Reactants Products 2 Li(s) + F 2(g) 2 Li. F(s) Note the 2 in front of the Li on the reactant side and the 2 in front of the Li. F on the product side are called a coefficients.

Synthesis Reaction 2 Li(s) + Br 2(l) 2 Li. Br (s) Lithium Bromide

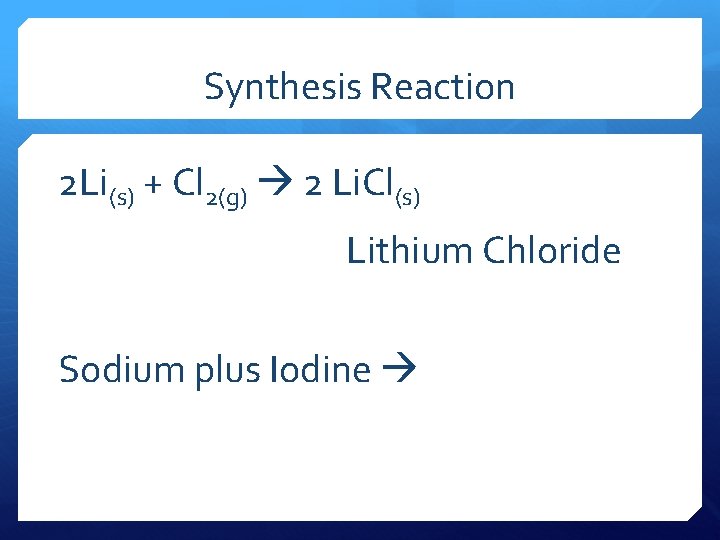
Synthesis Reaction 2 Li(s) + Cl 2(g) 2 Li. Cl(s) Lithium Chloride Sodium plus Iodine

Synthesis Reactions Na(s) + I 2(s)

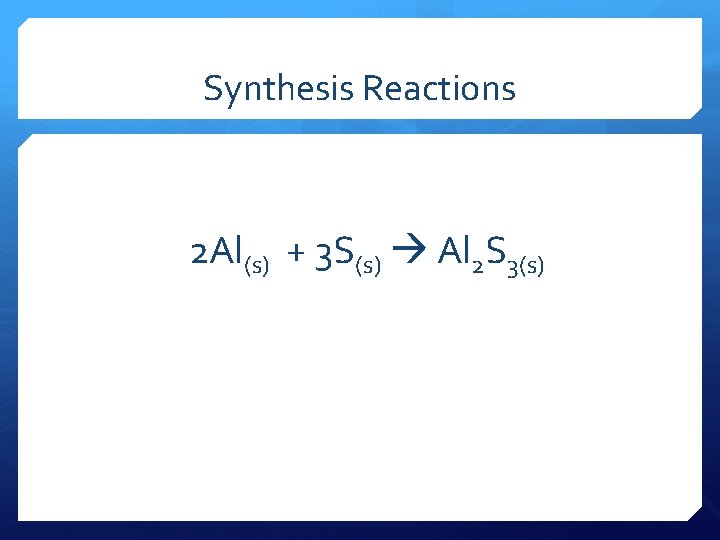
Synthesis Reactions 2 Al(s) + 3 S(s) Al 2 S 3(s)

Synthesis 2 Mg(s) + O 2(g) 2 Mg. O(s) Magnesium Oxide

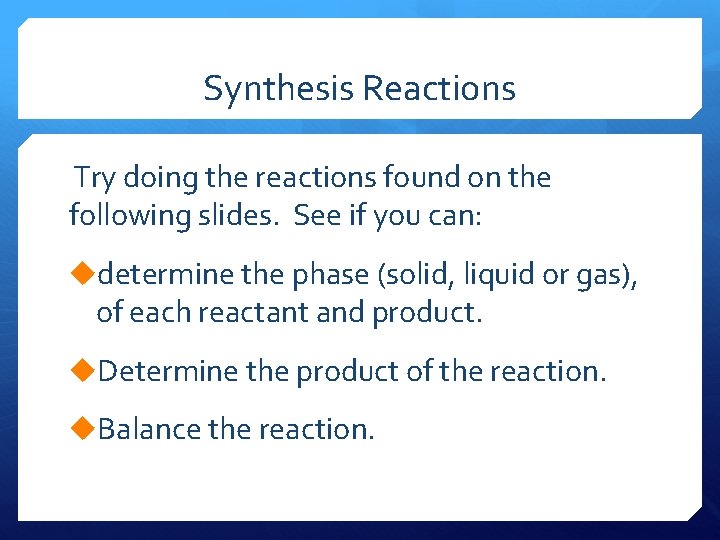
Synthesis Reactions Try doing the reactions found on the following slides. See if you can: udetermine the phase (solid, liquid or gas), of each reactant and product. u. Determine the product of the reaction. u. Balance the reaction.

Synthesis Reactions Magnesium plus oxygen

Synthesis Reactions Aluminum plus Selenium

Synthesis Reactions Calcium plus Nitrogen

Aluminum plus Phosphorus

Synthesis Reaction Barium plus Chlorine

Synthesis Reaction Gallium plus Arsenic

Synthesis Reaction Hydrogen plus Oxygen

Synthesis Reaction Carbon Dioxide plus water

Synthesis Reaction Calcium Oxide plus Water

Synthesis Reactions dinitrogen pentoxide plus water

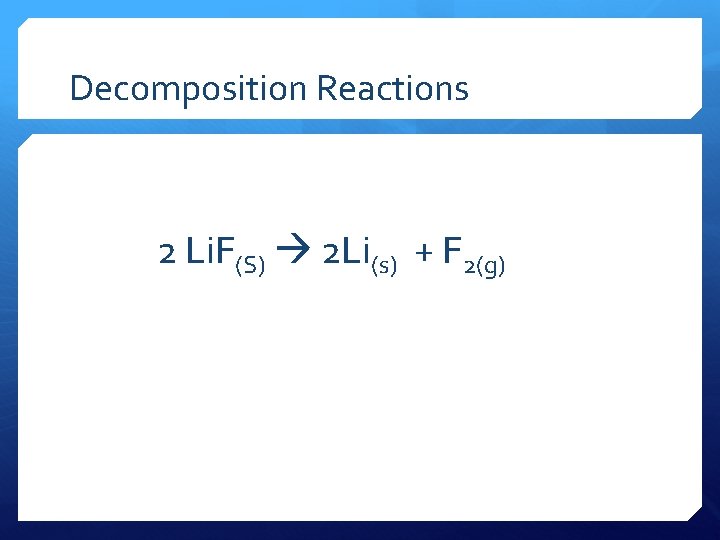
Decomposition Reactions 2 Li. F(S) 2 Li(s) + F 2(g)

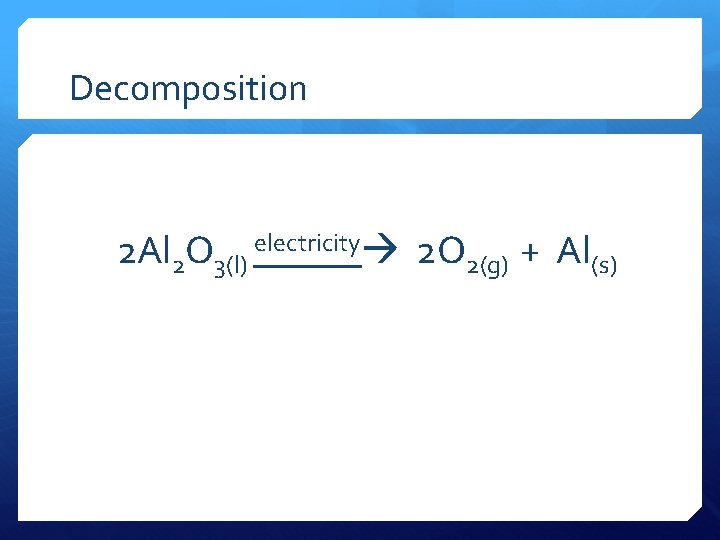
Decomposition 2 Al 2 O 3(l) electricity 2 O 2(g) + Al(s)

Decomposition H 2 CO 3 (aq) indicates that something is dissolved in water.

Decomposition Na. HCO 3 (s) --heat



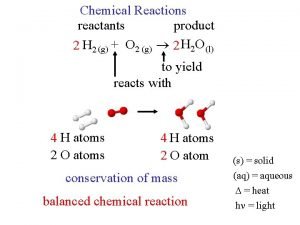
 Reactants and products
Reactants and products Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Synthesis reaction predicting products
Synthesis reaction predicting products Combination reaction equation
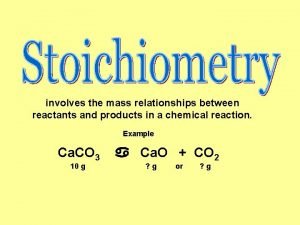
Combination reaction equation Stoichiometry is defined as the quantitative study of
Stoichiometry is defined as the quantitative study of Phet products reactants and leftovers
Phet products reactants and leftovers Enthalpy of reactants and products
Enthalpy of reactants and products Is photosynthesis a synthesis reaction
Is photosynthesis a synthesis reaction Cellular respiration reactants
Cellular respiration reactants Reactants, products, and leftovers
Reactants, products, and leftovers Elephant toothpaste chemical reaction
Elephant toothpaste chemical reaction Reactants and products
Reactants and products Reactants, products and leftovers
Reactants, products and leftovers Reactants minus products bond energies
Reactants minus products bond energies Reactant and products
Reactant and products Relationship between reactants and products
Relationship between reactants and products Enthalpy diagram worksheet
Enthalpy diagram worksheet Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Types of redox reactions
Types of redox reactions Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type 4 types of chemical reactions
4 types of chemical reactions