Types of Chemical Reactions Synthesis Reactions A B

Types of Chemical Reactions
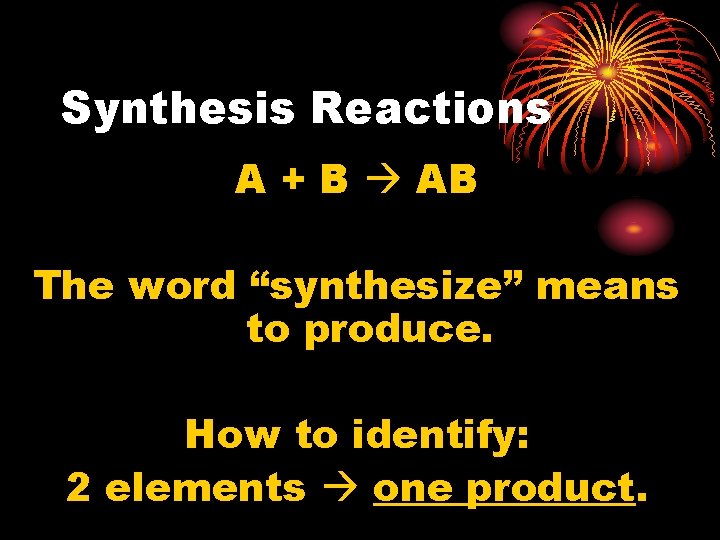
Synthesis Reactions A + B AB The word “synthesize” means to produce. How to identify: 2 elements one product.

Synthesis Reaction Ex. • 4 Fe + 3 O 2 2 Fe 2 O 3 • Iron + Oxygen Iron (III) Oxide

Decomposition Reactions AB A + B How to identify: they always have only one reactant.

Decomposition Rxn. Ex. • H 2 CO 3 CO 2 + H 2 O • Carbonic acid Carbon dioxide + Water
Single Replacement A + BC AC + B A single metal replaces another one in a compound.

Double Replacement AB + CD AD + CB The metals in two compounds switch places. (2 compounds 2 new compounds)

Combustion Organic cmpd. + O 2 CO 2 + H 2 O An organic compound is any compound containing C, H, and sometimes O.

Predicting Products of Reactions

Synthesis Two elements Write the formula correctly by balancing charges.

Synthesis cont. . . Nonmetal oxide + water Acid Combine atoms from both reactants. Start acid formula with H.
Decomposition Binary Compound Break into elements.

Decomposition cont. . . Metal Carbonate Metal oxide + CO 2

Single Replacement Metal + Compound(aq) Use “activity series” If the lone metal is higher than the one in the compound there will be a reaction. If not, write “N. R. ” in the products.

Ex. 2 Al(s) + 3 Pb(NO 3)2(aq) 3 Pb(s) + 2 Al(NO 3)3(aq) Al is more reactive (“fun”) and kicks Pb out of the “friendship”

Double Replacement Compound(aq) + Compound(aq) Use the Solubility Table from your book. If both products are SOLUBLE, write “N. R. ” in the products.

Combustion Organic + O 2 CO 2 + H 2 O Completely perfect combustion, but not reality.

Reactions in Aqueous Solution

Ions in Solution When ionic compounds dissolve in water they break apart: “Na. Cl(aq)” means Na+(aq) + Cl-(aq) “Ca. Cl 2(aq)” means Ca+2(aq) + 2 Cl-(aq)

Ionic Equations In a “complete ionic equation” you split the aqueous compounds into their ions: Na. Cl(aq) + Ag. NO 3(aq) Na. NO 3(aq) + Ag. Cl(s) Becomes Na+(aq) + Cl-(aq) + Ag+(aq) + NO 3 -(aq) Na+(aq) + NO 3 -(aq) + Ag. Cl(s)

Ionic Equations Some of the ions don’t change from one side to the other (Na+ and NO 3 -). They are “spectator ions”.
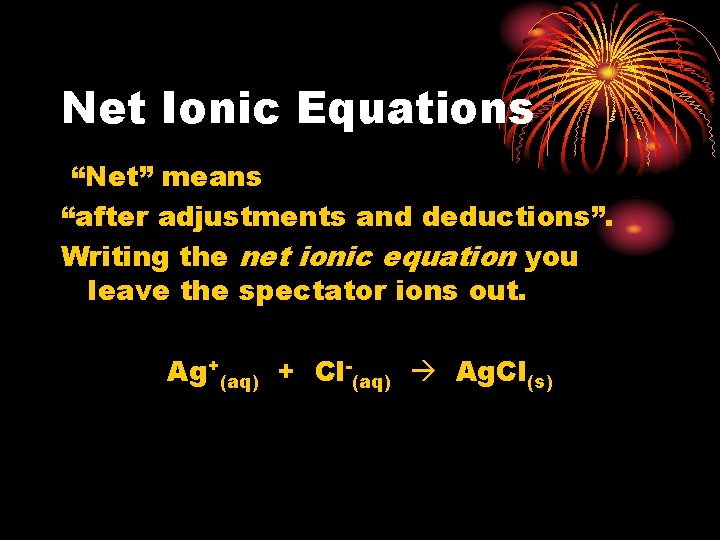
Net Ionic Equations “Net” means “after adjustments and deductions”. Writing the net ionic equation you leave the spectator ions out. Ag+(aq) + Cl-(aq) Ag. Cl(s)

Examples Balance these, then write the complete ionic equation: Pb(Cl. O 4)2(aq) + Na. I(aq) Pb. I 2(s) + Na. Cl. O 4(aq) Zn(s) + HCl(aq) Zn. Cl 2(aq) + H 2(g)
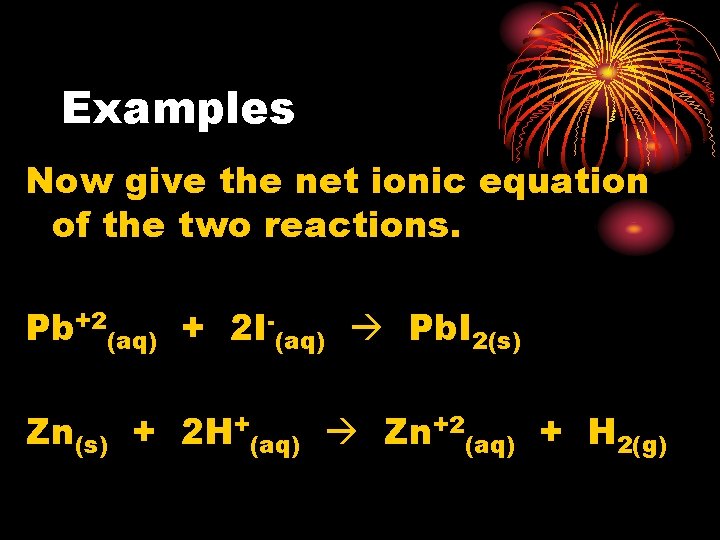
Examples Now give the net ionic equation of the two reactions. Pb+2(aq) + 2 I-(aq) Pb. I 2(s) Zn(s) + 2 H+(aq) Zn+2(aq) + H 2(g)

Keep in Mind When you have the SAME coefficient for ALL of the reactants AND products, reduce them to ONE.
- Slides: 25