Types of Chemical Reactions Synthesis Decomposition Combustion Single
Types of Chemical Reactions • Synthesis • Decomposition • Combustion • Single Replacement • Double Replacement

Chemistry Joke Little Willie was a chemist. Little Willie is no more. For what he thought was H Was really H 2 SO 4.
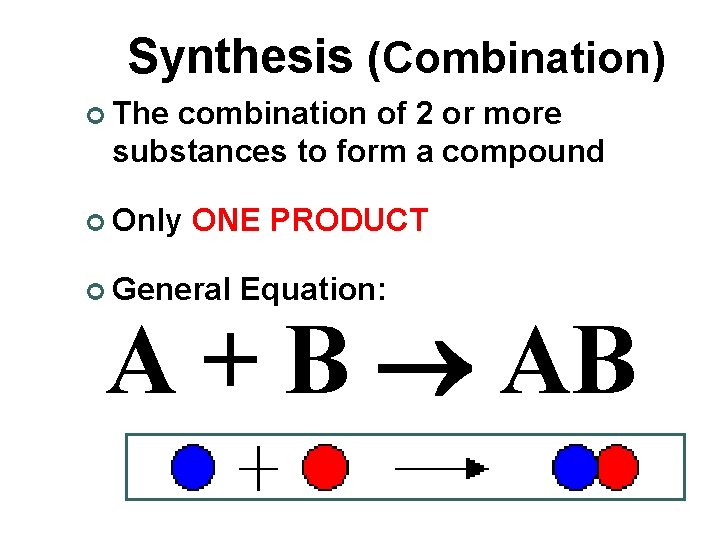
Synthesis (Combination) ¢ The combination of 2 or more substances to form a compound ¢ Only ONE PRODUCT ¢ General Equation: A + B AB

Synthesis Example H 2(g) + Cl 2(g) 2 HCl(g) When 2 elements combine, they combine as ions!!! + - • H and Cl become HCl. • Then…the equation is balanced.
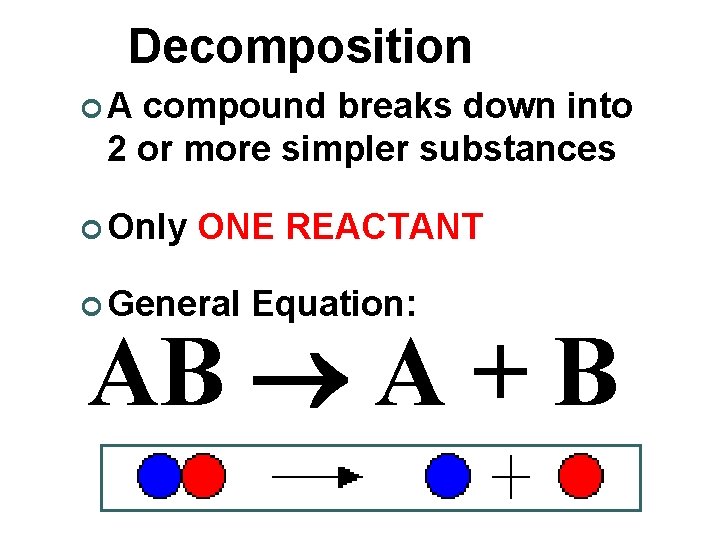
Decomposition ¢A compound breaks down into 2 or more simpler substances ¢ Only ONE REACTANT ¢ General Equation: AB A + B
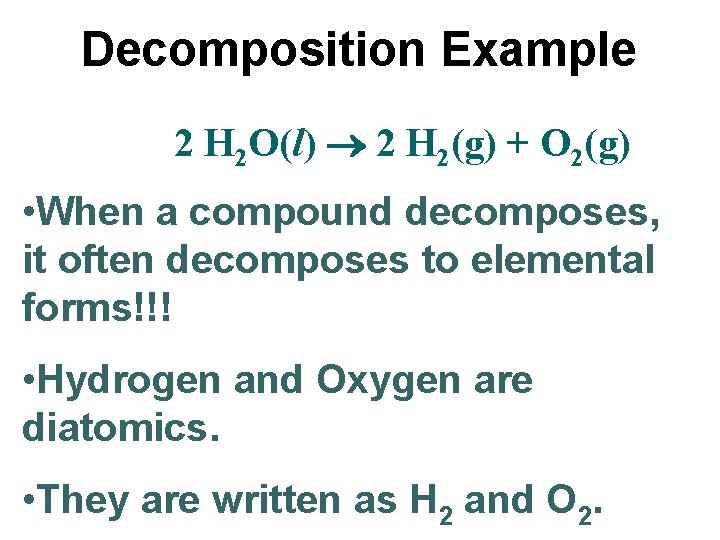
Decomposition Example 2 H 2 O(l) 2 H 2(g) + O 2(g) • When a compound decomposes, it often decomposes to elemental forms!!! • Hydrogen and Oxygen are diatomics. • They are written as H 2 and O 2.

Combustion ¢ Definition: reaction with O 2 that releases energy. ¢ Usually, the burning of a molecular compound containing carbon, hydrogen, and/or oxygen in the presence of O 2 to produce heat, CO 2, and water. ¢ General Equation: Hydrocarbon + O 2 CO 2 + H 2 O CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

Incomplete Combustion ¢ Produces Carbon Monoxide and Water instead of Carbon Dioxide and Water. ¢ Example: 2 C 2 H 2(g) + O 2(g) 4 CO(g) + 2 H 2 O(g)

Single Replacement ¢ One element replaces another in a compound l Metal replaces metal (+) l Nonmetal replaces nonmetal (-) A + BC AC + B
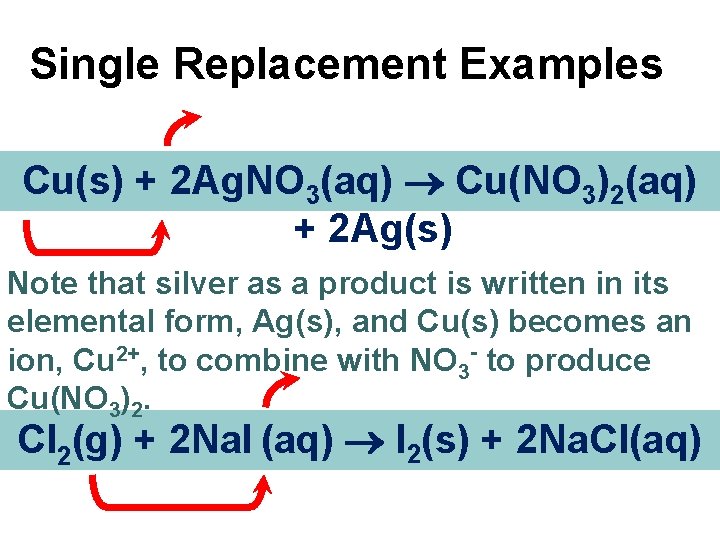
Single Replacement Examples Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s) Note that silver as a product is written in its elemental form, Ag(s), and Cu(s) becomes an ion, Cu 2+, to combine with NO 3 - to produce Cu(NO 3)2. Cl 2(g) + 2 Na. I (aq) I 2(s) + 2 Na. Cl(aq)

Activity Series Will a reaction occur? A metal will only replace another metal below it on the activity series. So, will this reaction happen? Ca + 2 Li. OH Ca(OH)2 + 2 Li NO!!!

Chemistry Joke Q: How often do chemistry teachers give tests? A: Periodically!
- Slides: 12