Types of Chemical Reactions Synthesis Composition Reactions Two











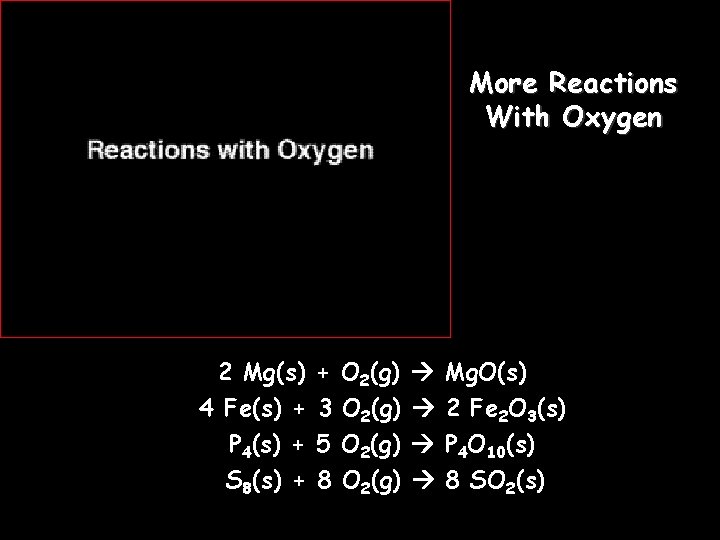



- Slides: 25

Types of Chemical Reactions

Synthesis (Composition) Reactions Two or more substances combine to form a new compound. A + X AX q q Reaction of elements with oxygen and sulfur Reactions of metals with Halogens Synthesis Reactions with Oxides There are others not covered here!

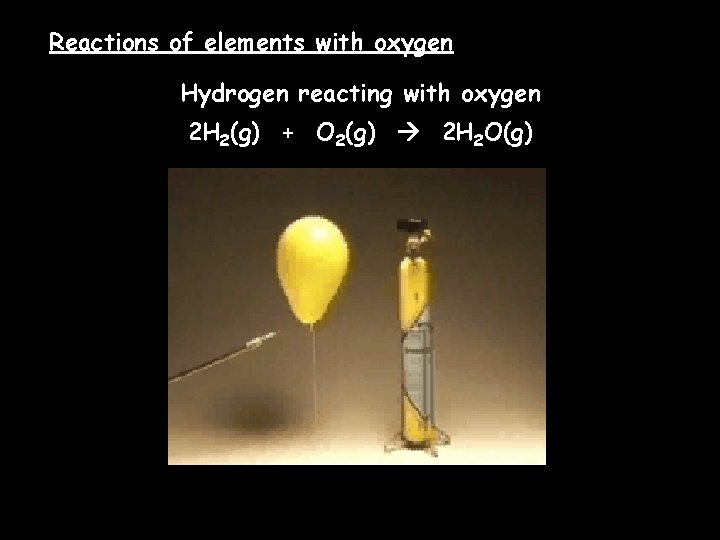
Reactions of elements with oxygen Hydrogen reacting with oxygen 2 H 2(g) + O 2(g) 2 H 2 O(g)

…a metal with a halogen: Reaction of aluminum and bromine 2 Al(s) + 3 Br 2(g) 2 Al. Br 3(s)

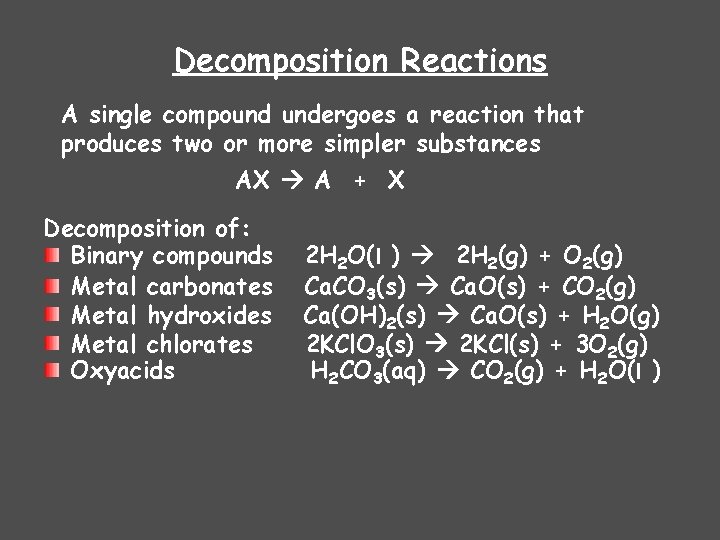
Decomposition Reactions A single compound undergoes a reaction that produces two or more simpler substances AX A + X Decomposition of: Binary compounds Metal carbonates Metal hydroxides Metal chlorates Oxyacids 2 H 2 O(l ) 2 H 2(g) + O 2(g) Ca. CO 3(s) Ca. O(s) + CO 2(g) Ca(OH)2(s) Ca. O(s) + H 2 O(g) 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) H 2 CO 3(aq) CO 2(g) + H 2 O(l )

Decomposition of Nitrogen Triiodide 2 NI 3(s) N 2(g) + 3 I 2(g)

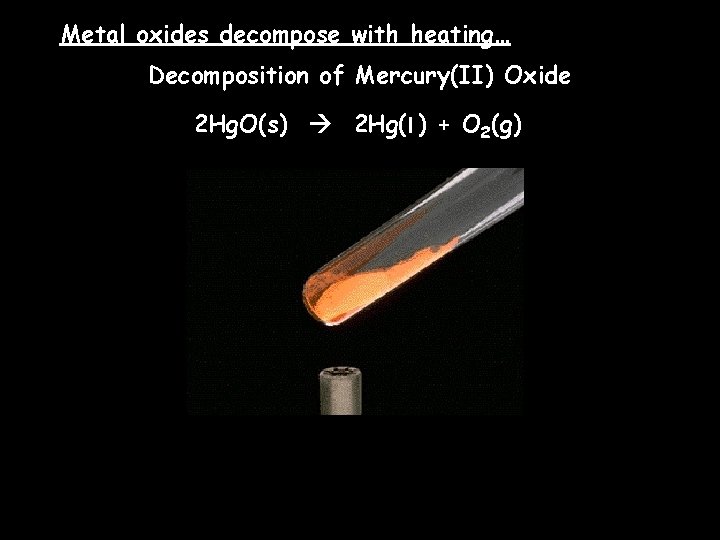
Metal oxides decompose with heating… Decomposition of Mercury(II) Oxide 2 Hg. O(s) 2 Hg(l ) + O 2(g)

Some decomposition needs catalysis… Catalyzed Decomposition of Hydrogen Peroxide 2 H 2 O 2(l ) 2 H 2 O(l ) + O 2(g)

Single Replacement Reactions Red. Ox Reactons A + BX AX + B BX + Y BY + X Replacement of: Ø Ø Metals by another metal Hydrogen in water by a metal Hydrogen in an acid by a metal Halogens by more active halogens

Redox (short for reduction–oxidation reaction) is a chemical reaction in which the oxidation states of atoms are changed. Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.

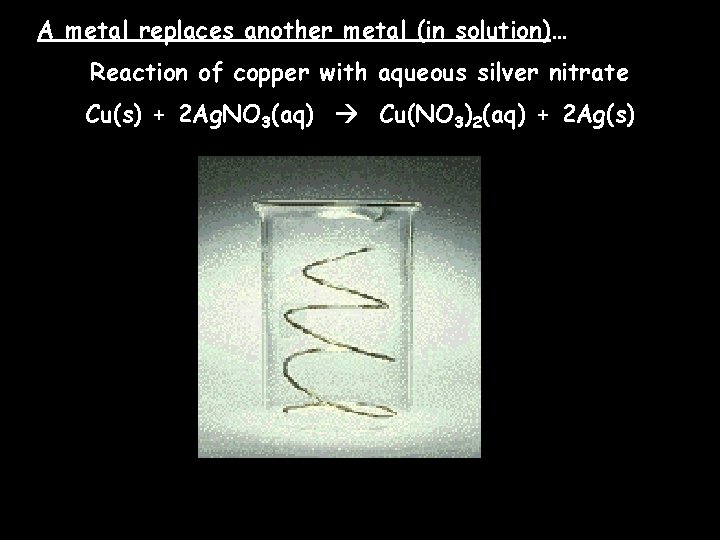
A metal replaces another metal (in solution)… Reaction of copper with aqueous silver nitrate Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s)

A metal replaces another metal (no solution needed!)… Thermite reaction 2 Al(s) + Fe 2 O 3(s) Al 2 O 3(s) + 2 Fe(s)

An active metal replaces hydrogen in water… Reaction of potassium with water 2 K(s) + 2 H 2 O(l ) 2 KOH(aq) + H 2(g)

A metal replaces hydrogen in an acid… Magnesium in Hydrochloric Acid Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g)

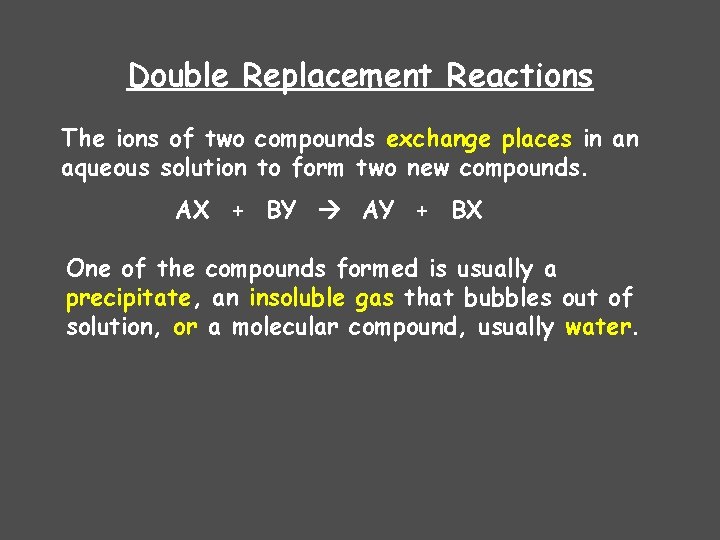
Double Replacement Reactions The ions of two compounds exchange places in an aqueous solution to form two new compounds. AX + BY AY + BX One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of solution, or a molecular compound, usually water.

Double replacement forming a precipitate… Lead(II) nitrate reacts with potassium iodide Pb(NO 3)2(aq) + 2 KI(aq) Pb. I 2(s) + 2 KNO 3(aq)

Double replacement forming water… Reaction of Hydrochloric acid and Sodium hydroxide HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l )

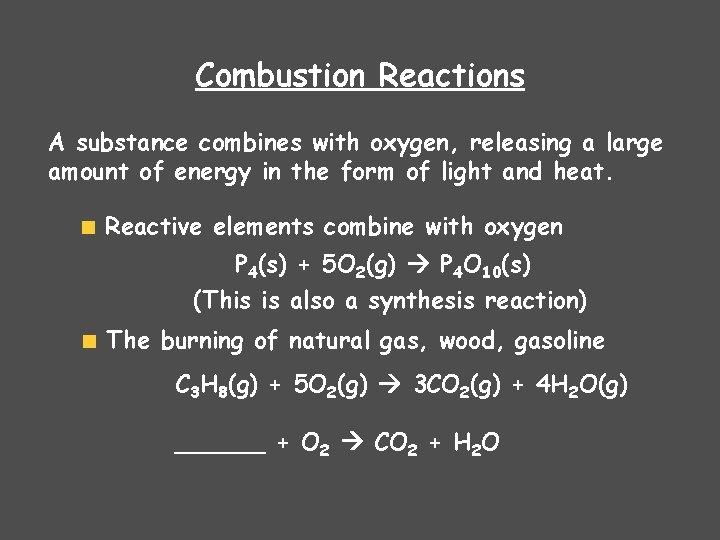
Combustion Reactions A substance combines with oxygen, releasing a large amount of energy in the form of light and heat. Reactive elements combine with oxygen P 4(s) + 5 O 2(g) P 4 O 10(s) (This is also a synthesis reaction) The burning of natural gas, wood, gasoline C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) ______ + O 2 CO 2 + H 2 O

An element burning in oxygen… Reaction of white phosphorus and oxygen P 4(s) + 5 O 2(g) P 4 O 10(s)
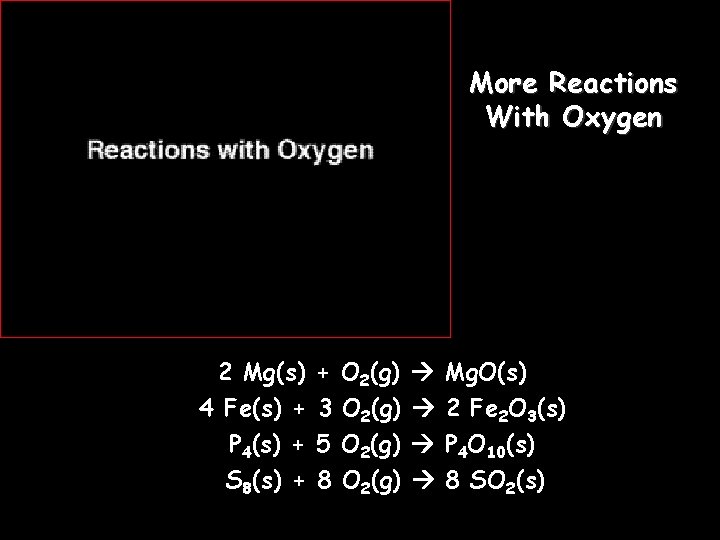
More Reactions With Oxygen 2 Mg(s) 4 Fe(s) + P 4(s) + S 8(s) + + 3 5 8 O 2(g) Mg. O(s) 2 Fe 2 O 3(s) P 4 O 10(s) 8 SO 2(s)

Energy for space travel… Hydrogen and oxygen reacting 2 H 2(g) + O 2(g) 2 H 2 O(g) + energy

Burning of natural gas… Combustion of Propane C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g)

Burning of an alcohol… Combustion of methanol CH 3 OH(l ) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

The Activity Series of the Metals Lithium Potassium Calcium Sodium Magnesium Aluminum Zinc Chromium Iron Nickel Lead Hydrogen Bismuth Copper Mercury Silver Platinum Gold Metals can replace other metals provided that they are above the metal that they are trying to replace. Metals above hydrogen can replace hydrogen in acids. Metals from sodium upward can replace hydrogen in water

The Activity Series of the Halogens Fluorine Chlorine Bromine Iodine Halogens can replace other halogens in compounds, provided that they are above the halogen that they are trying to replace. 2 Na. Cl(s) + F 2(g) 2 Na. F(s) ? ? ? + Cl 2(g) Mg. Cl 2(s) + Br 2(g) No ? ? ? Reaction