Types of Chemical Reactions Synthesis Combustion Decomposition and
Types of Chemical Reactions Synthesis, Combustion, Decomposition and Replacement
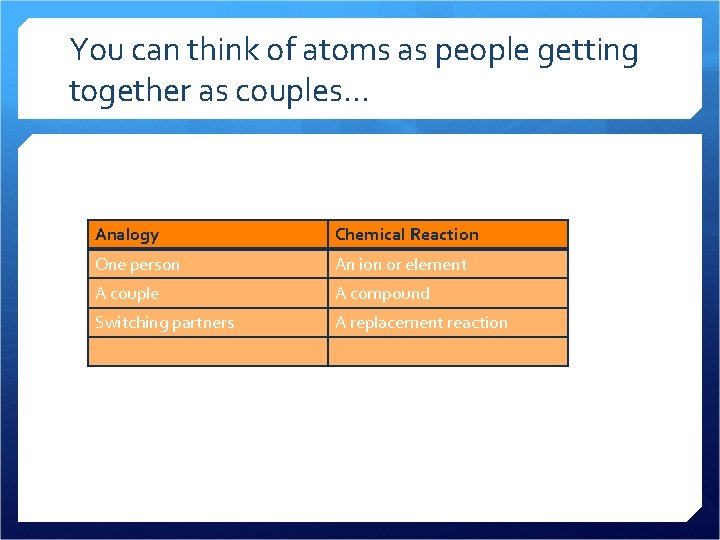
You can think of atoms as people getting together as couples. . . Analogy Chemical Reaction One person An ion or element A couple A compound Switching partners A replacement reaction
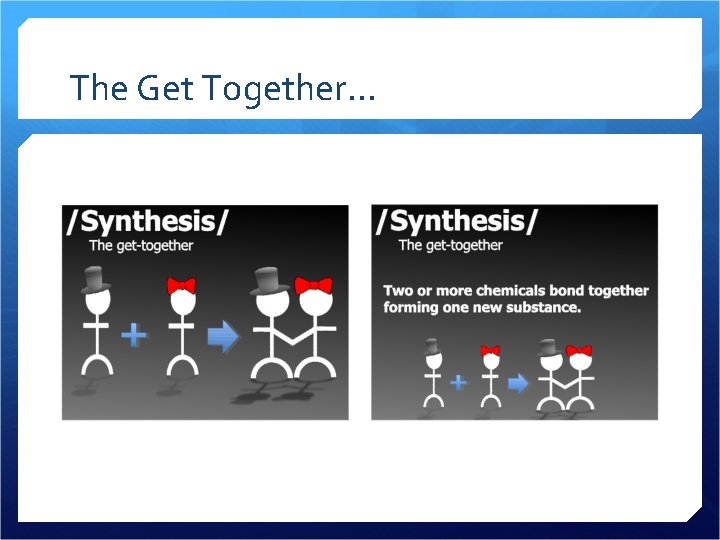
The Get Together…
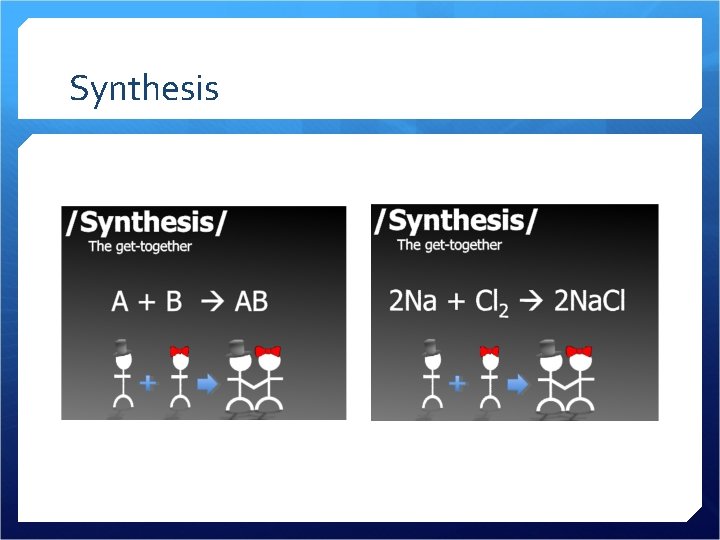
Synthesis
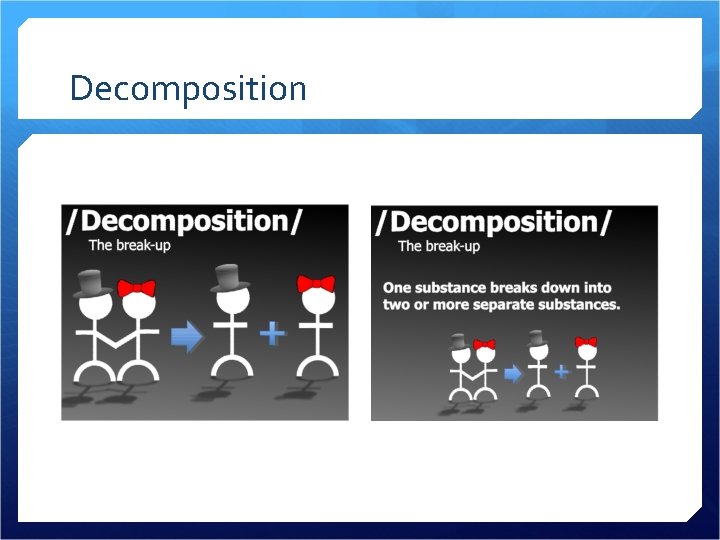
Decomposition
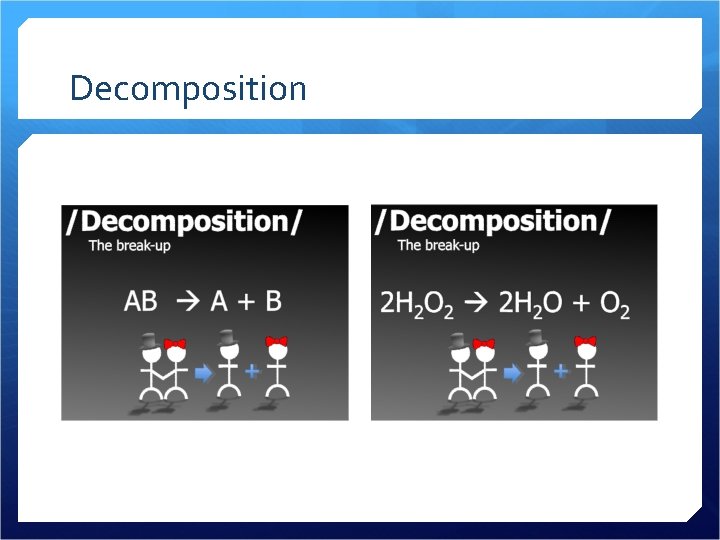
Decomposition
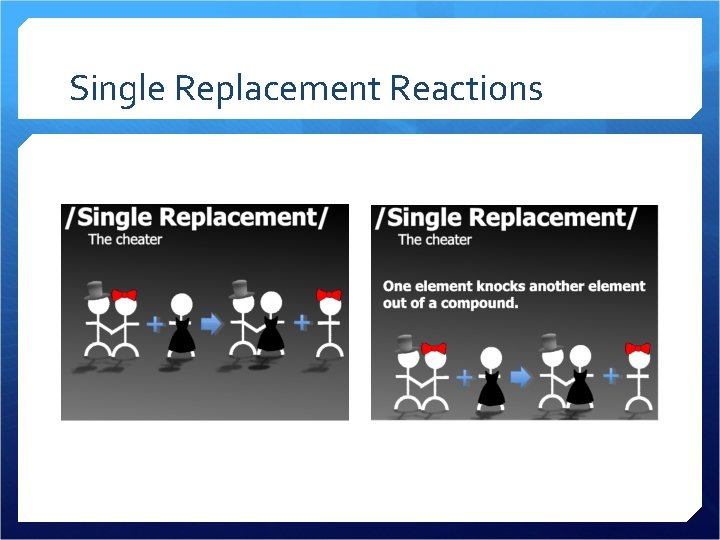
Single Replacement Reactions
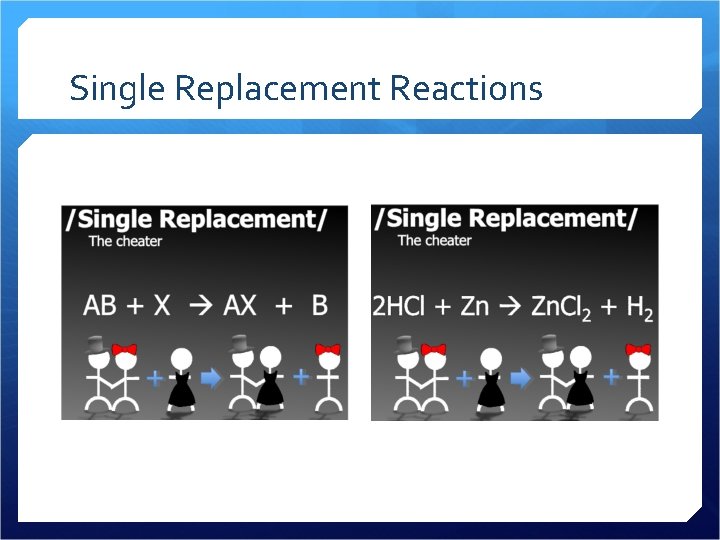
Single Replacement Reactions
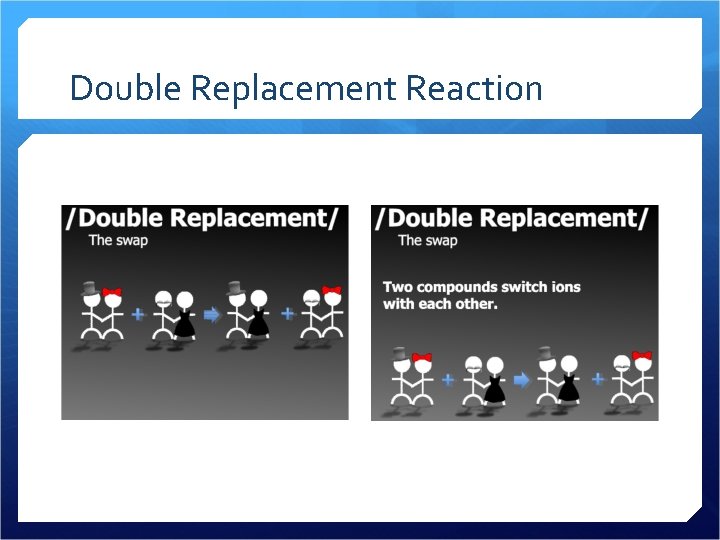
Double Replacement Reaction
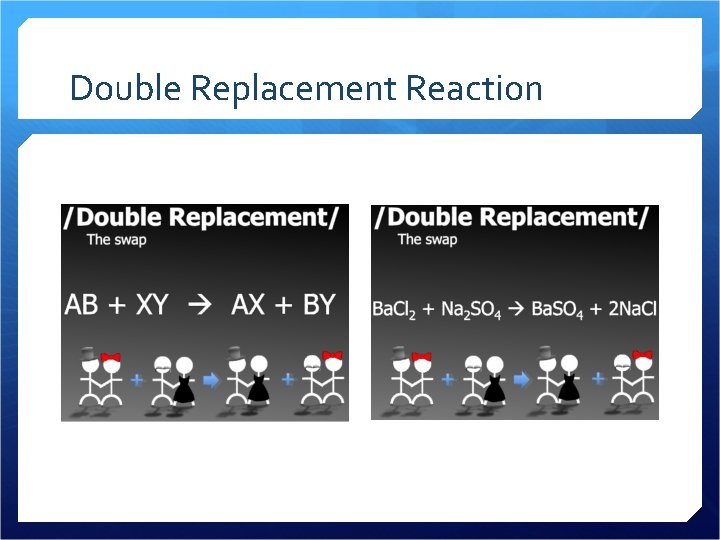
Double Replacement Reaction
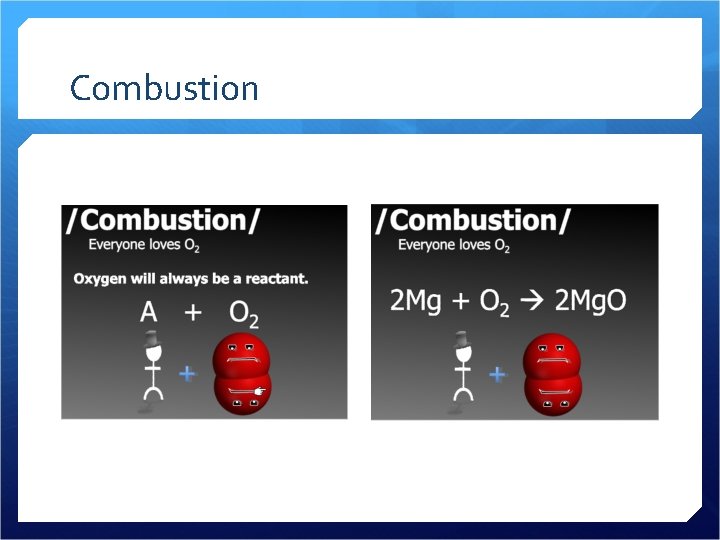
Combustion
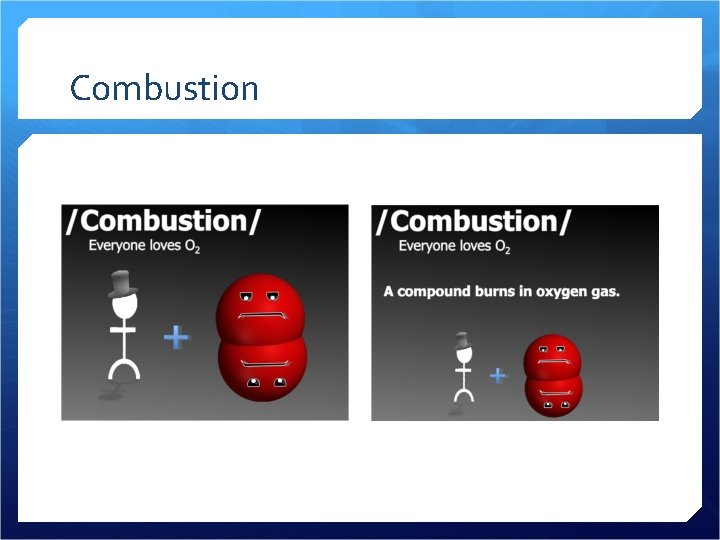
Combustion
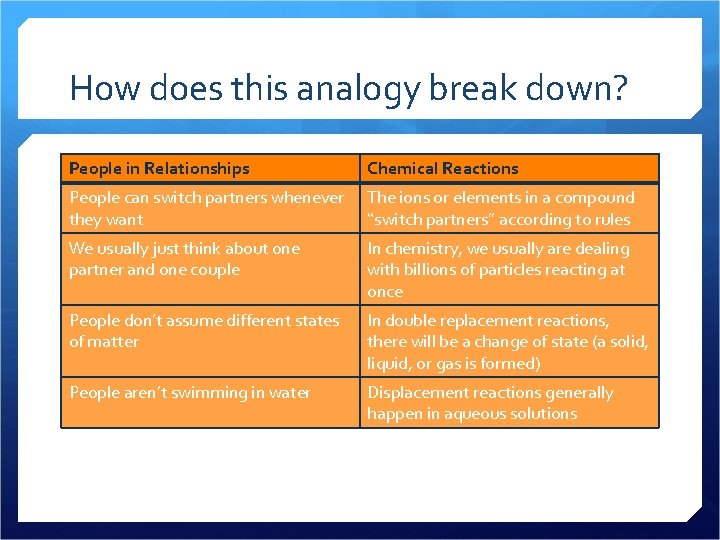
How does this analogy break down? People in Relationships Chemical Reactions People can switch partners whenever they want The ions or elements in a compound “switch partners” according to rules We usually just think about one partner and one couple In chemistry, we usually are dealing with billions of particles reacting at once People don’t assume different states of matter In double replacement reactions, there will be a change of state (a solid, liquid, or gas is formed) People aren’t swimming in water Displacement reactions generally happen in aqueous solutions

Let’s look at the reactions in more detail Synthesis Decomposition Single-Replacement Double-Replacement Combustion
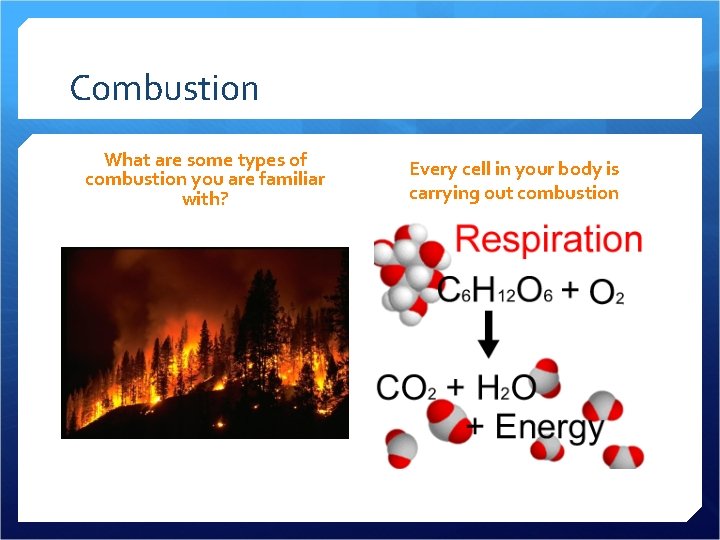
Combustion What are some types of combustion you are familiar with? Every cell in your body is carrying out combustion

Combustion Oxygen combines with a metal, non-metal, or compound Creates a metal oxide, non-metal oxide, or 2 or more oxides Releases energy – heat, light Can be a slower process too…
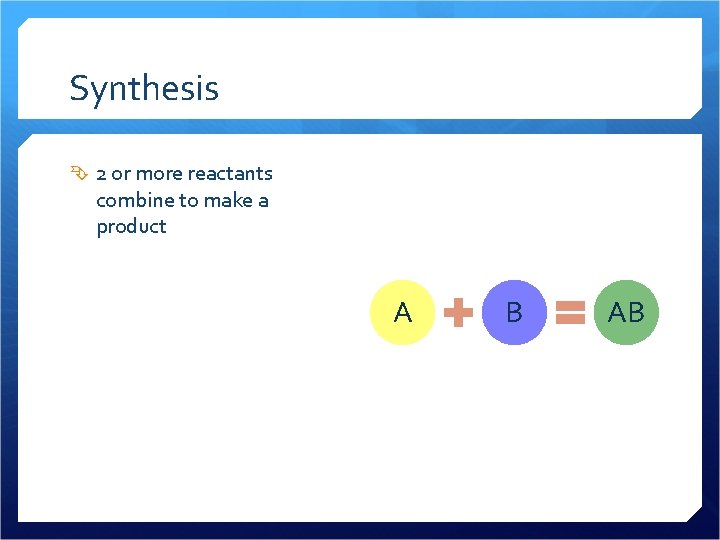
Synthesis 2 or more reactants combine to make a product A B AB
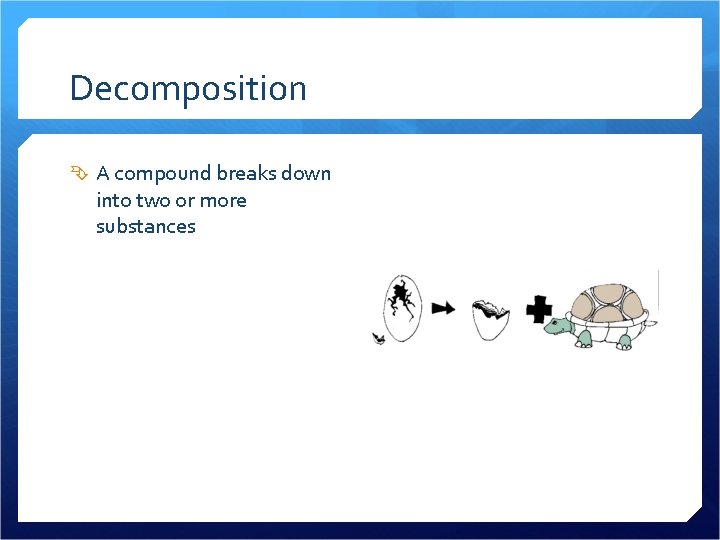
Decomposition A compound breaks down into two or more substances
Single Replacement A metal replaces a hydrogen A metal replaces another metal We use an activity series to predict which metals are “stronger” and can knock out other metals from compounds
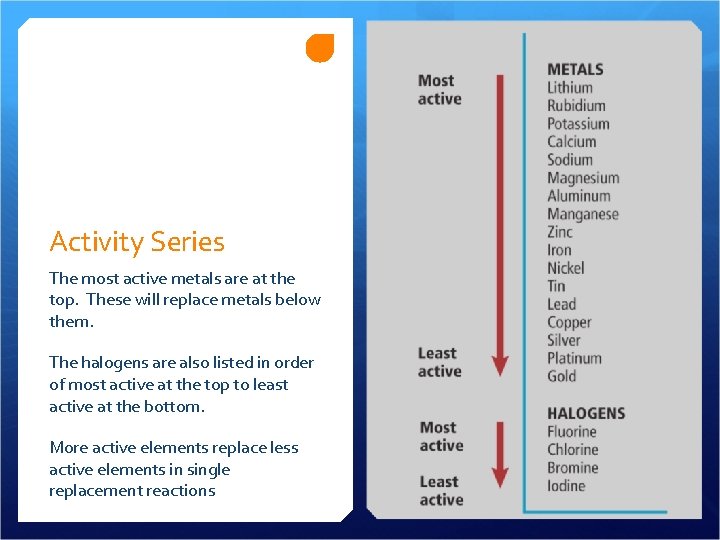
Activity Series The most active metals are at the top. These will replace metals below them. The halogens are also listed in order of most active at the top to least active at the bottom. More active elements replace less active elements in single replacement reactions
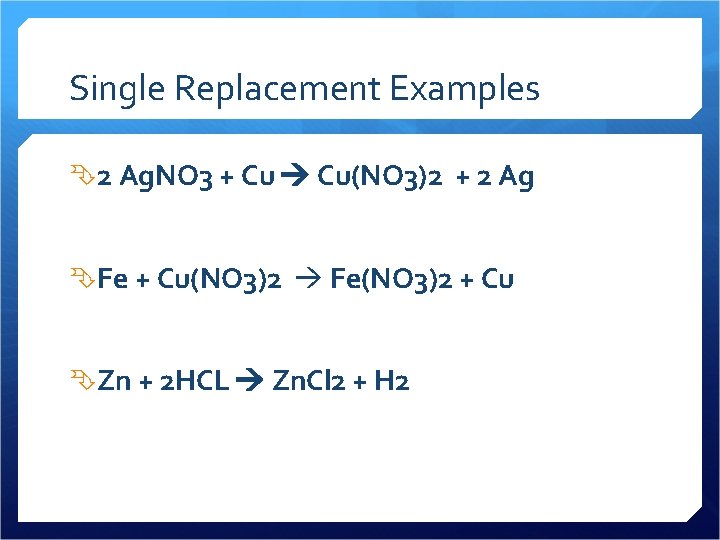
Single Replacement Examples 2 Ag. NO 3 + Cu Cu(NO 3)2 + 2 Ag Fe + Cu(NO 3)2 Fe(NO 3)2 + Cu Zn + 2 HCL Zn. Cl 2 + H 2
Double Replacement Reactions Produces a precipitate, liquid or gas All four of the ions switch partners Aqueous solution
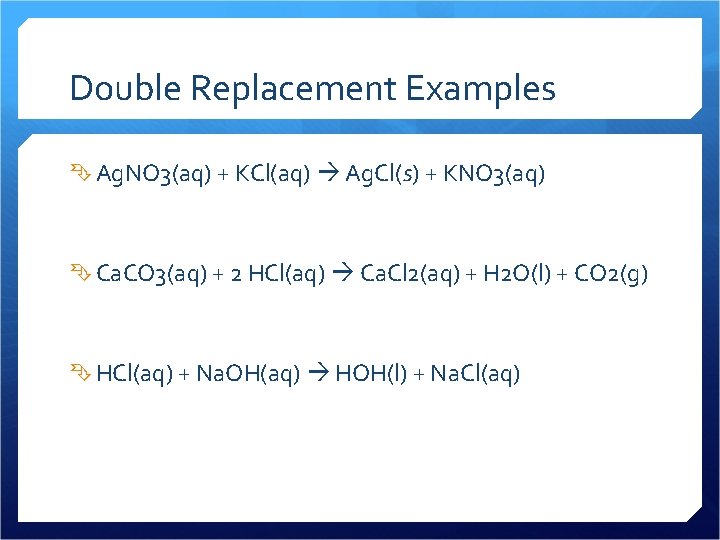
Double Replacement Examples Ag. NO 3(aq) + KCl(aq) Ag. Cl(s) + KNO 3(aq) Ca. CO 3(aq) + 2 HCl(aq) Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) HCl(aq) + Na. OH(aq) HOH(l) + Na. Cl(aq)
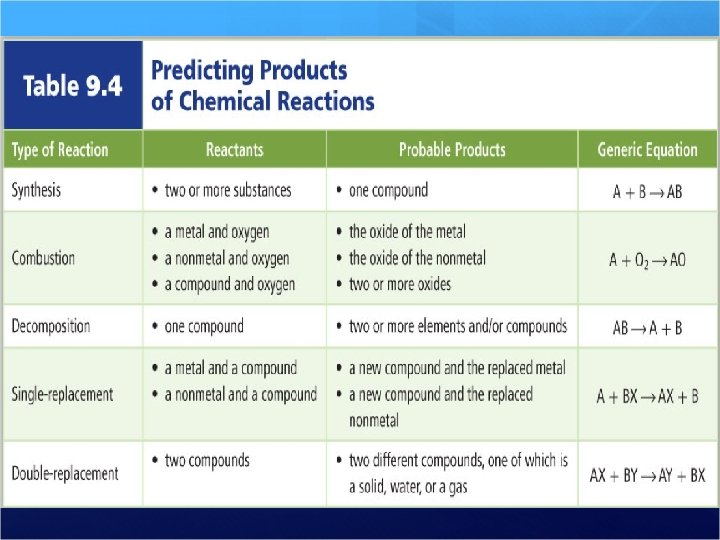

Steps to determine type of reaction Write the chemical equation How to identify a reaction Be systematic – it will help you identify the reaction type and the products! Determine what is happening in the reaction Use the table to identify the type of reaction Check your answer by comparing the chemical equation to the generic equation
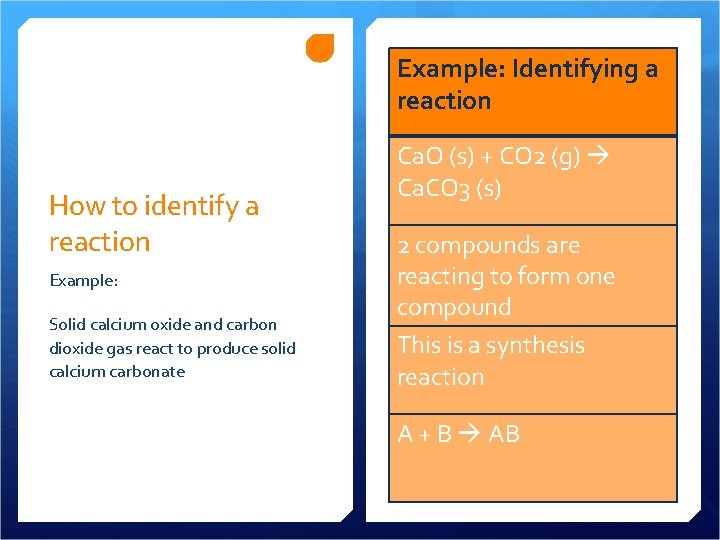
Example: Identifying a reaction How to identify a reaction Example: Solid calcium oxide and carbon dioxide gas react to produce solid calcium carbonate Ca. O (s) + CO 2 (g) Ca. CO 3 (s) 2 compounds are reacting to form one compound This is a synthesis reaction A + B AB
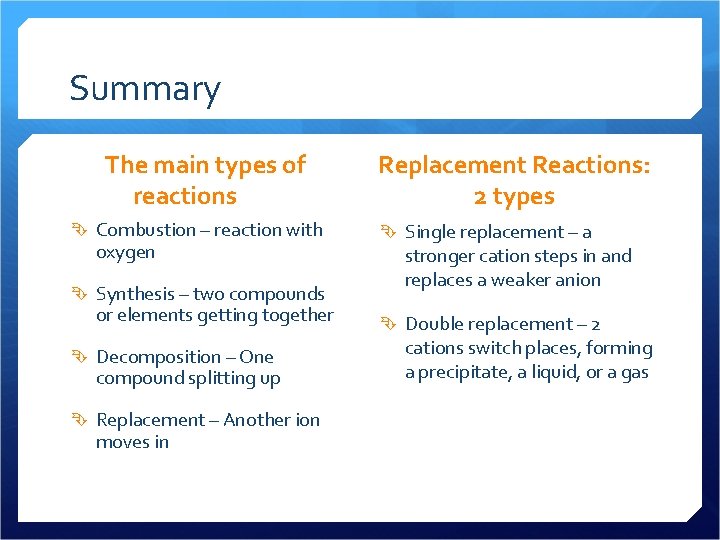
Summary The main types of reactions Combustion – reaction with oxygen Synthesis – two compounds or elements getting together Decomposition – One compound splitting up Replacement – Another ion moves in Replacement Reactions: 2 types Single replacement – a stronger cation steps in and replaces a weaker anion Double replacement – 2 cations switch places, forming a precipitate, a liquid, or a gas

You try it! Work on these examples together in pairs. Write the reaction Balance the equation Classify the reaction Be prepared to present your results on the board! Magnesium and Hydrochloric acid form Magnesium Chloride and Hydrogen Carbon Dioxide and Water form Carbonic Acid (H 2 CO 3) Silver Nitrate and Sodium Chloride form Silver Chloride and Sodium Nitrate Heating sodium bicarbonate (Na. HCO 3) releases water and carbon dioxide and sodium carbonate

You try it! Magnesium and Hydrochloric acid form Magnesium Chloride and Hydrogen: Mg + HCl Mg. Cl + H 2 (skeleton equation) 2 Mg + 2 HCl 2 Mg. Cl + H 2 (balanced equation) Single replacement (reaction type) Carbon Dioxide and Water form Carbonic Acid (H 2 CO 3): CO 2 + H 2 O H 2 CO 3 (skeleton equation) CO 2 + H 2 O H 2 CO 3 (it’s already balanced!) Synthesis (reaction type)

You try it! Silver Nitrate and Sodium Chloride form Silver Chloride and Sodium Nitrate Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 (skeleton equation) Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 (already balanced) Double replacement (reaction type) Heating sodium bicarbonate releases water and carbon dioxide and sodium carbonate Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 (skeleton equation) 2 Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 (balanced equation) Decomposition (reaction type)
- Slides: 30