Types of Chemical Reactions Synthesis combination Two or
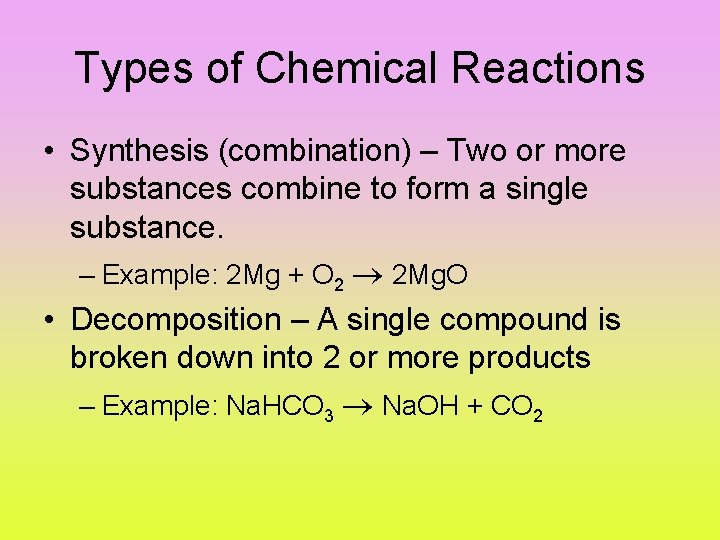
Types of Chemical Reactions • Synthesis (combination) – Two or more substances combine to form a single substance. – Example: 2 Mg + O 2 2 Mg. O • Decomposition – A single compound is broken down into 2 or more products – Example: Na. HCO 3 Na. OH + CO 2
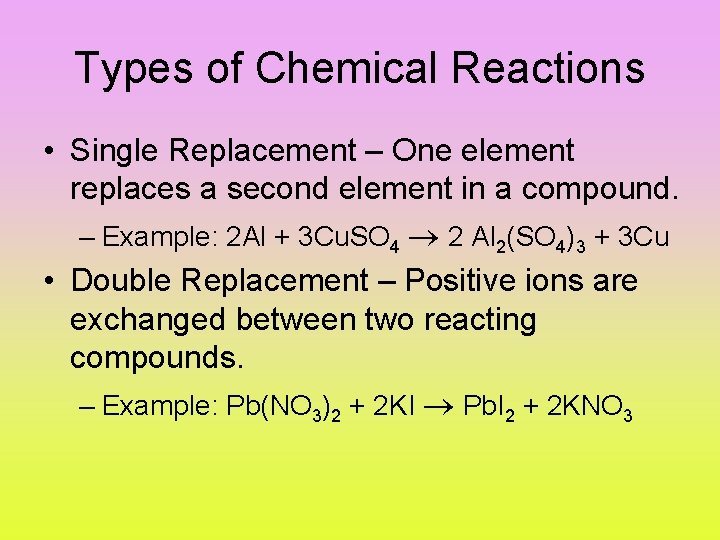
Types of Chemical Reactions • Single Replacement – One element replaces a second element in a compound. – Example: 2 Al + 3 Cu. SO 4 2 Al 2(SO 4)3 + 3 Cu • Double Replacement – Positive ions are exchanged between two reacting compounds. – Example: Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3
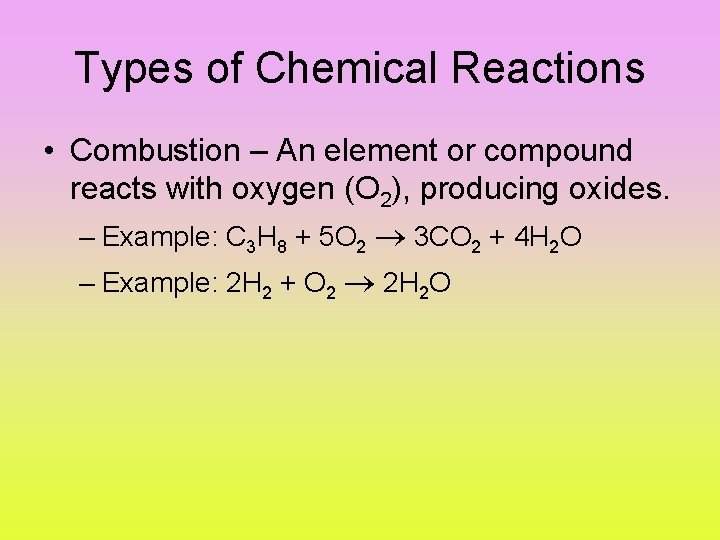
Types of Chemical Reactions • Combustion – An element or compound reacts with oxygen (O 2), producing oxides. – Example: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O – Example: 2 H 2 + O 2 2 H 2 O
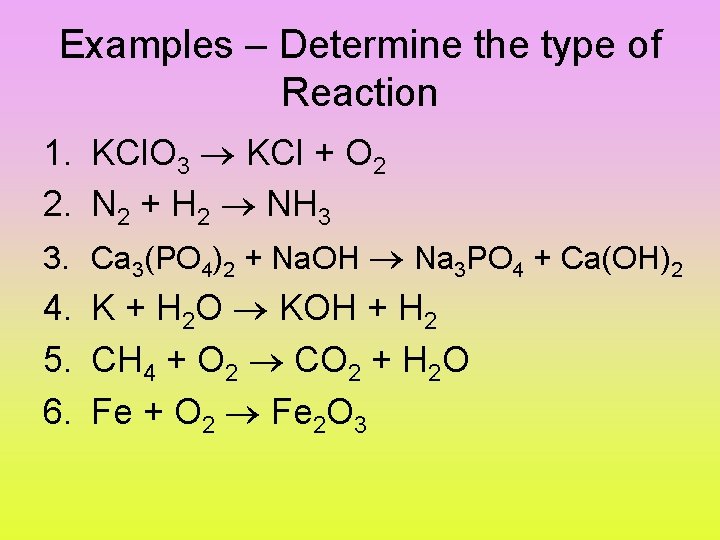
Examples – Determine the type of Reaction 1. KCl. O 3 KCl + O 2 2. N 2 + H 2 NH 3 3. Ca 3(PO 4)2 + Na. OH Na 3 PO 4 + Ca(OH)2 4. K + H 2 O KOH + H 2 5. CH 4 + O 2 CO 2 + H 2 O 6. Fe + O 2 Fe 2 O 3
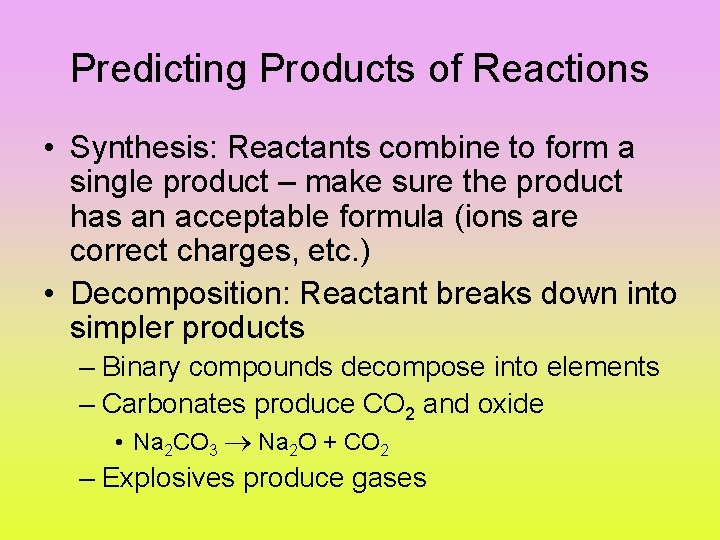
Predicting Products of Reactions • Synthesis: Reactants combine to form a single product – make sure the product has an acceptable formula (ions are correct charges, etc. ) • Decomposition: Reactant breaks down into simpler products – Binary compounds decompose into elements – Carbonates produce CO 2 and oxide • Na 2 CO 3 Na 2 O + CO 2 – Explosives produce gases
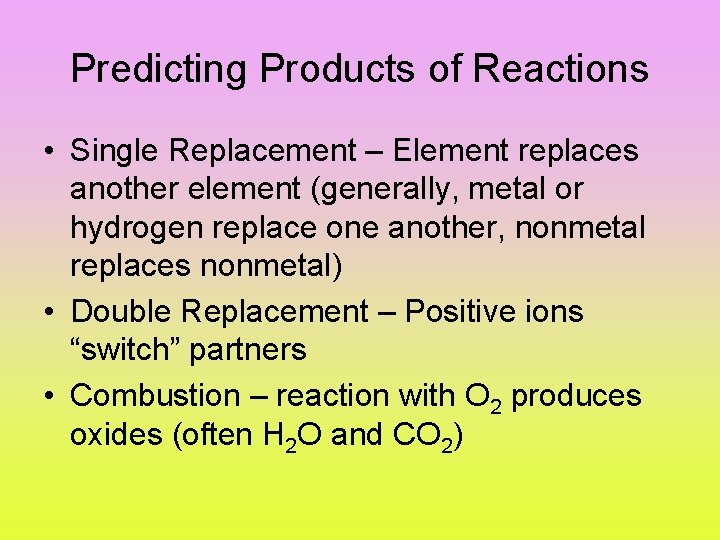
Predicting Products of Reactions • Single Replacement – Element replaces another element (generally, metal or hydrogen replace one another, nonmetal replaces nonmetal) • Double Replacement – Positive ions “switch” partners • Combustion – reaction with O 2 produces oxides (often H 2 O and CO 2)
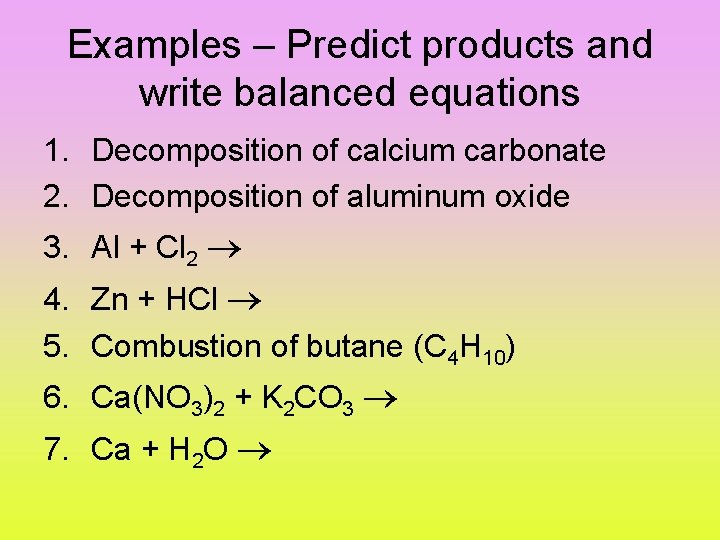
Examples – Predict products and write balanced equations 1. Decomposition of calcium carbonate 2. Decomposition of aluminum oxide 3. Al + Cl 2 4. Zn + HCl 5. Combustion of butane (C 4 H 10) 6. Ca(NO 3)2 + K 2 CO 3 7. Ca + H 2 O
- Slides: 7