Types of Chemical Reactions States of matter symbols
Types of Chemical Reactions
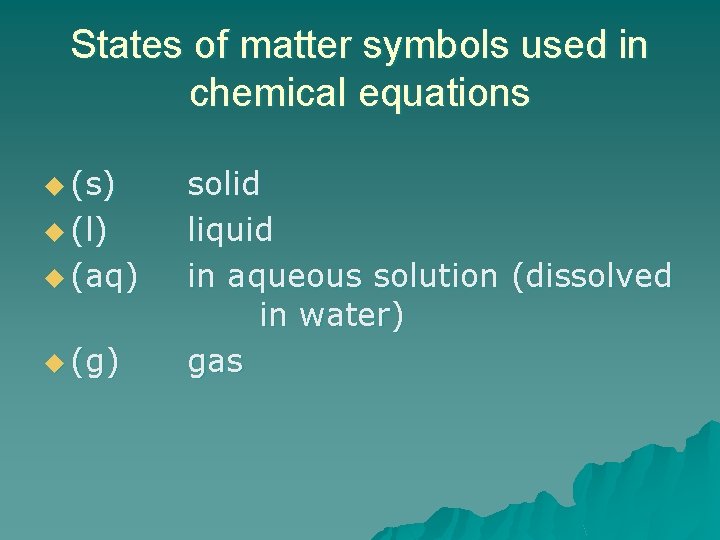
States of matter symbols used in chemical equations u (s) u (l) u (aq) u (g) solid liquid in aqueous solution (dissolved in water) gas

Types of Chemical Reactions 1. 2. 3. 4. 5. Synthesis Decomposition Single Displacement Double Displacement Combustion

Synthesis Two or more reactants combine to form a single product A + B AB General Form Example: 2 Al(s) + 3 Br 2(l) 2 Al. Br 3(s)
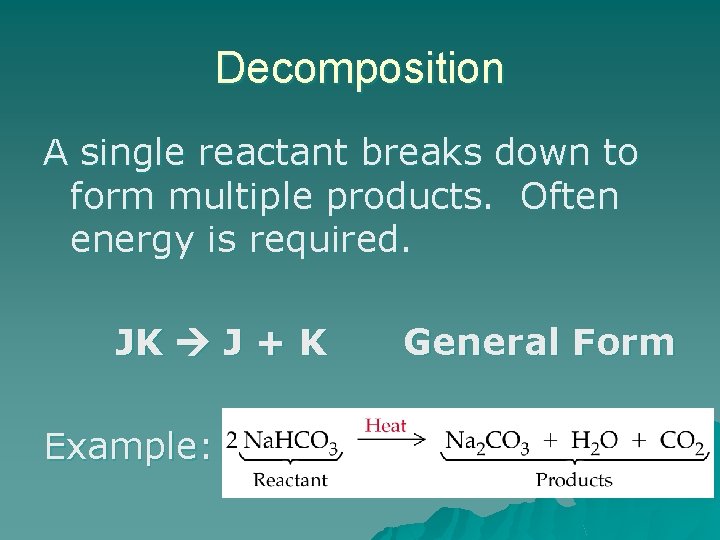
Decomposition A single reactant breaks down to form multiple products. Often energy is required. JK J + K Example: General Form
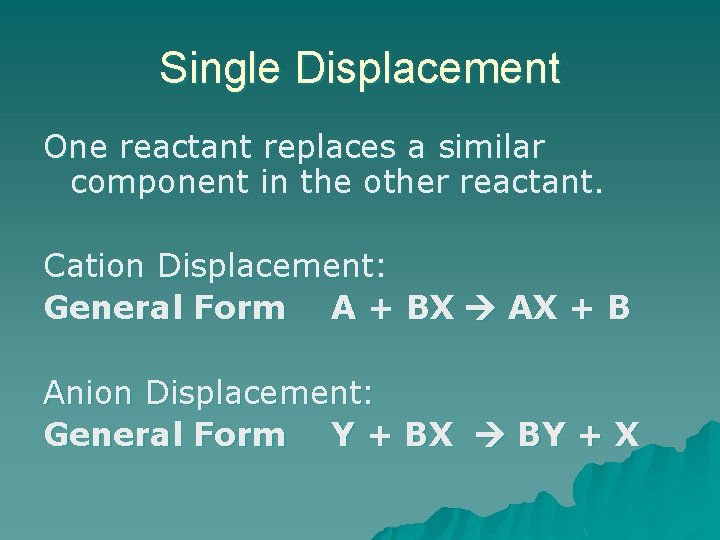
Single Displacement One reactant replaces a similar component in the other reactant. Cation Displacement: General Form A + BX AX + B Anion Displacement: General Form Y + BX BY + X

Double Displacement Two cations and two anions swap components and rearrange. General Form: AX + B Y AY + B X Example: Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3
Combustion A substance combines with oxygen, releasing large amounts of energy in the form of light and heat. Usually combustion rxns yield the products carbon dioxide and water. General Form: Cx. Hy + O 2 CO 2 + H 2 O Example: The metabolism of glucose. C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l)
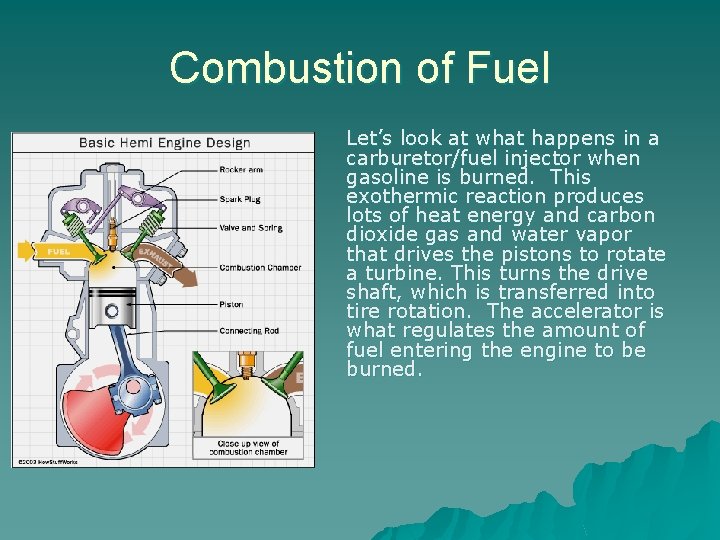
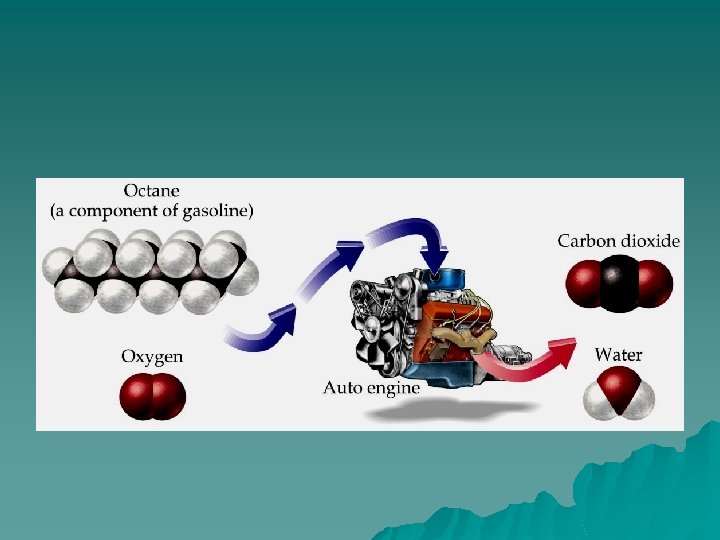
Combustion of Fuel Let’s look at what happens in a carburetor/fuel injector when gasoline is burned. This exothermic reaction produces lots of heat energy and carbon dioxide gas and water vapor that drives the pistons to rotate a turbine. This turns the drive shaft, which is transferred into tire rotation. The accelerator is what regulates the amount of fuel entering the engine to be burned.
- Slides: 10