Types of Chemical Reactions Solution Stoichiometry Chapter 4










































- Slides: 65

Types of Chemical Reactions & Solution Stoichiometry Chapter 4

Aqueous Solutions Water is the dissolving medium, or solvent.

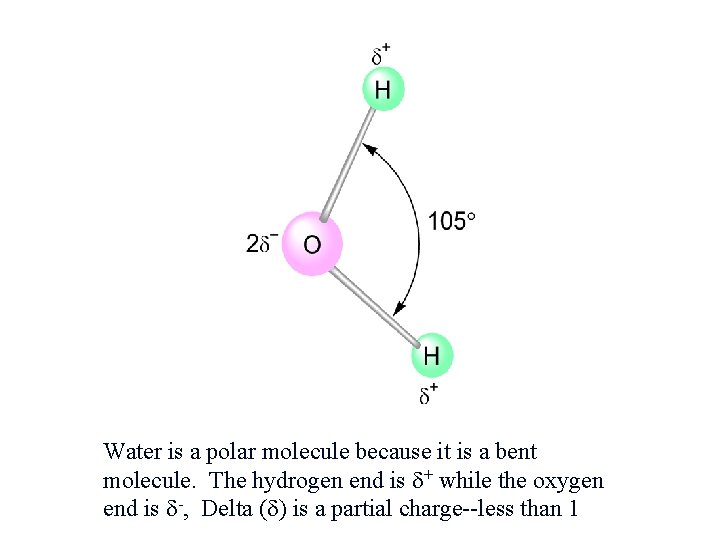
Some Properties of Water is able to dissolve so many substances because: - Water is “bent” or V-shaped. - 105° bond angle - The O-H bonds are covalent. - Water is a polar molecule. - Hydration occurs when salts dissolve in water.

Water is a polar molecule because it is a bent molecule. The hydrogen end is + while the oxygen end is -, Delta ( ) is a partial charge--less than 1.

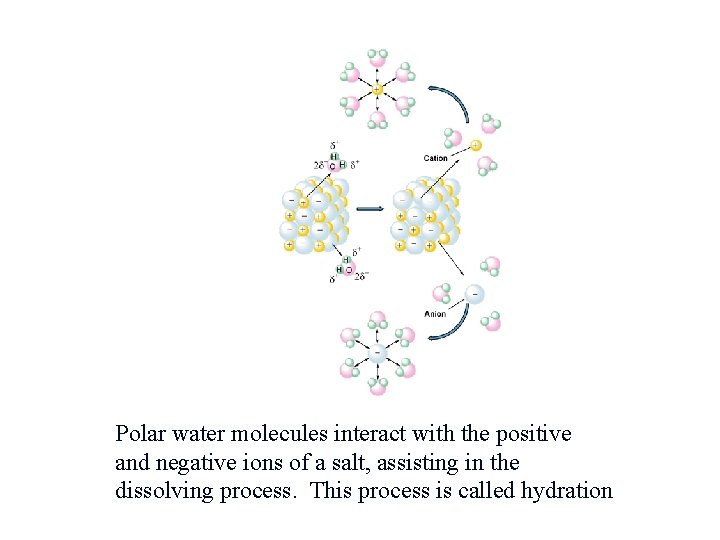
Polar water molecules interact with the positive and negative ions of a salt, assisting in the dissolving process. This process is called hydration.

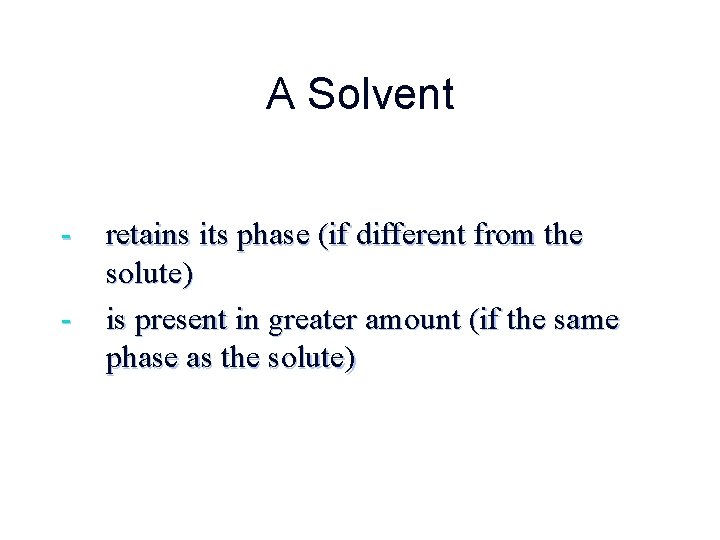
A Solute - dissolves in water (or other “solvent”) - changes phase (if different from the solvent) - is present in lesser amount (if the same phase as the solvent)

A Solvent - retains its phase (if different from the solute) - is present in greater amount (if the same phase as the solute)

Solubility The general rule for solubility is: “Like dissolves like. ” Polar water molecules can dissolve other polar molecules such as alcohol and, also, ionic substances such as Na. Cl. Nonpolar molecules can dissolve other nonpolar molecules but not polar or ionic substances. Gasoline can dissolve grease.

Miscibility Miscible -- two substances that will mix together in any proportion to make a solution. Alcohol and water are miscible because they are both polar and form hydrogen bonds. (compounds that have hydrogen bonding readily dissolve) Immiscible -- two substances that will not dissolve in each other. Oil and vinegar are immiscible because oil is nonpolar and vinegar is polar.

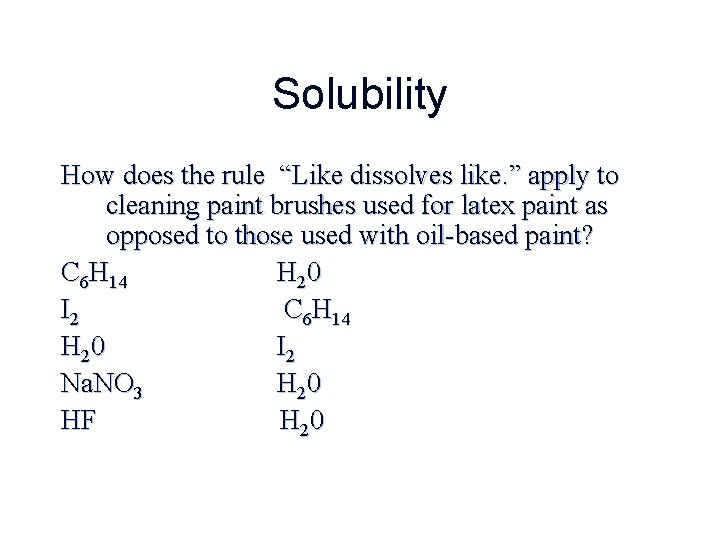
Solubility How does the rule “Like dissolves like. ” apply to cleaning paint brushes used for latex paint as opposed to those used with oil-based paint? C 6 H 14 H 20 I 2 Na. NO 3 H 20 HF H 20

Electrolytes & Nonelectrolytes An electrolyte is a material that dissolves in water to give a solution that conducts an electric current. A nonelectrolyte is a substance which, when dissolved in water, gives a nonconducting solution.

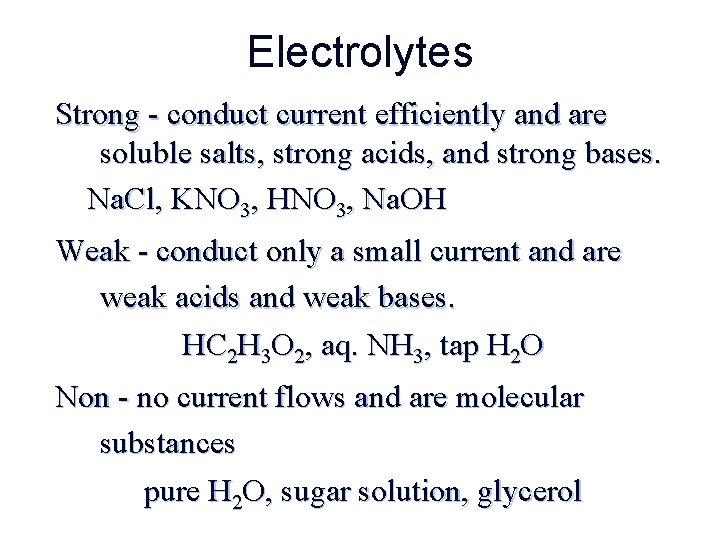
Electrolytes Strong - conduct current efficiently and are soluble salts, strong acids, and strong bases. Na. Cl, KNO 3, HNO 3, Na. OH Weak - conduct only a small current and are weak acids and weak bases. HC 2 H 3 O 2, aq. NH 3, tap H 2 O Non - no current flows and are molecular substances pure H 2 O, sugar solution, glycerol

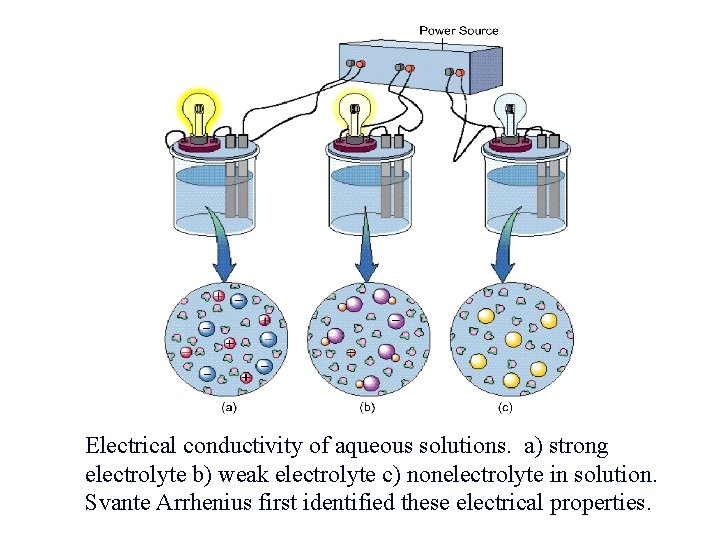
Electrical conductivity of aqueous solutions. a) strong electrolyte b) weak electrolyte c) nonelectrolyte in solution. Svante Arrhenius first identified these electrical properties.

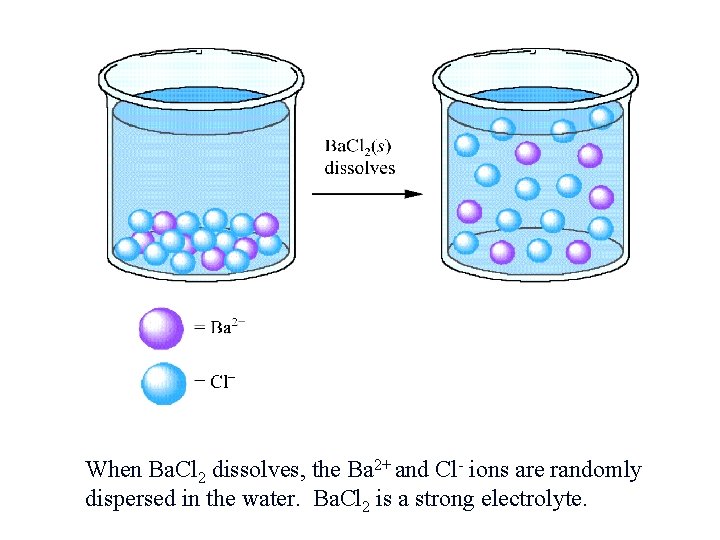
When Ba. Cl 2 dissolves, the Ba 2+ and Cl- ions are randomly dispersed in the water. Ba. Cl 2 is a strong electrolyte.

Acids Strong acids - dissociate completely (~100 %) to produce H+ in solution HCl, H 2 SO 4, HNO 3, HBr, HI, & HCl. O 4 Weak acids - dissociate to a slight extent (~ 1 %) to give H+ in solution HC 2 H 3 O 2, HCOOH, HNO 2, & H 2 SO 3

HCl is completely ionized and is a strong electrolyte.

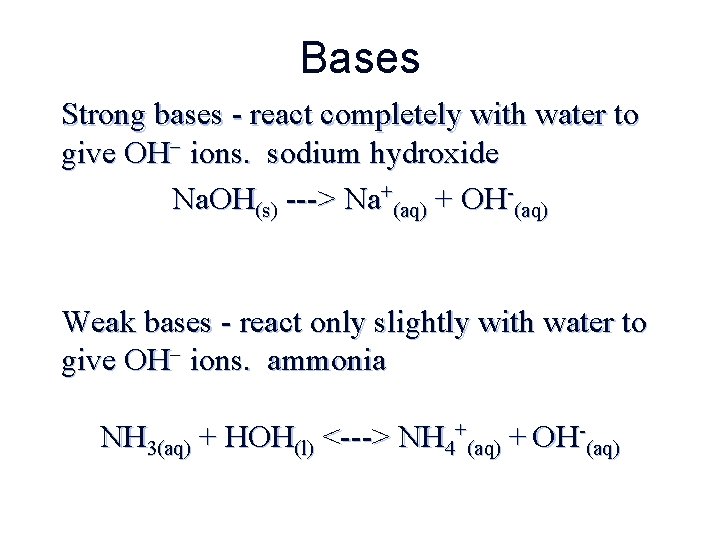
Bases Strong bases - react completely with water to give OH ions. sodium hydroxide Na. OH(s) ---> Na+(aq) + OH-(aq) Weak bases - react only slightly with water to give OH ions. ammonia NH 3(aq) + HOH(l) <---> NH 4+(aq) + OH-(aq)

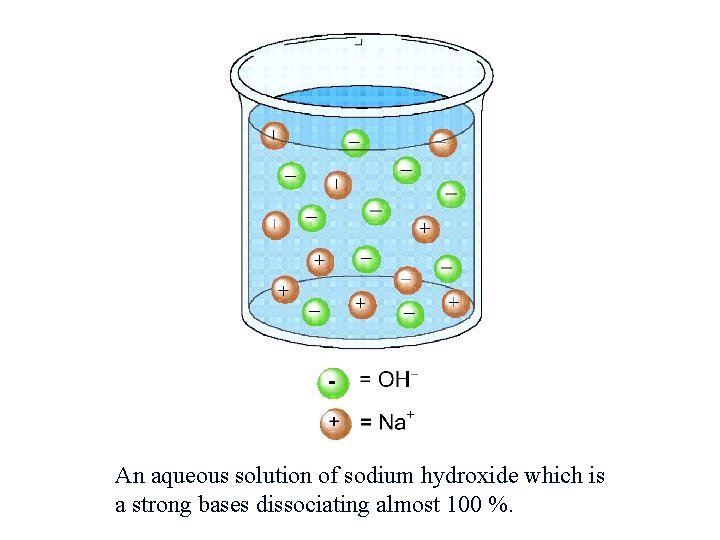
An aqueous solution of sodium hydroxide which is a strong bases dissociating almost 100 %.

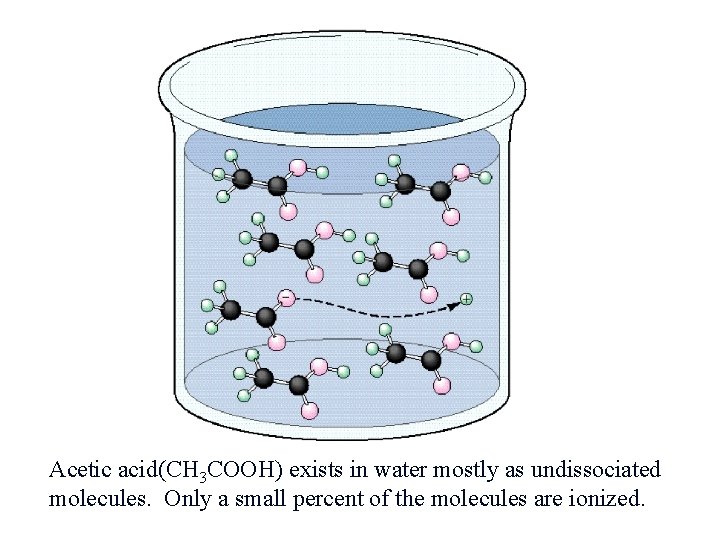
Acetic acid(CH 3 COOH) exists in water mostly as undissociated molecules. Only a small percent of the molecules are ionized.

Write the equation of the dissolving of the following compounds. Ca. Cl 2 HCl Fe(NO 3)3 KBr (NH 4)2 Cr 2 O 7

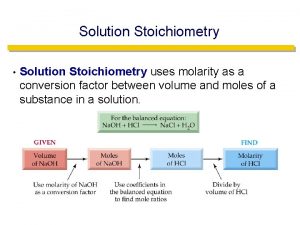
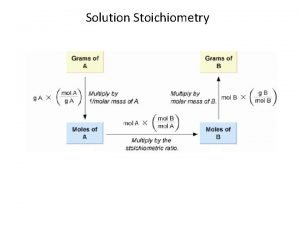
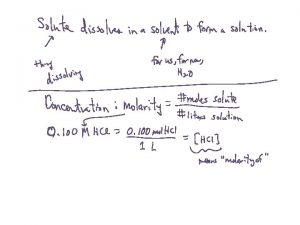
Molarity (M) = moles of solute per volume of solution in liters: 1. Calculate the molarity of a solution prepared by dissolving 11. 5 g of solid Na. OH in enough water to make 1. 50 L of solution. 2. Calculate the molarity of a solution prepared by dissolving 1. 56 g of gaseous HCl in enough water to make 26. 8 m. L of solution .


Calculating moles in a solution How many moles of Co(NO 3)2 are present in 25. 00 m. L of a 0. 75 M Co(NO 3)2 solution? How many moles of nitrate ions are present in 25. 00 m. L of a 0. 75 M Co(NO 3)2 solution?


Calculating moles in a solution Calculate the moles of each of the ions in 40. 00 ml of. 20 M Na 2 CO 3 solution. Calculate the moles of potassium ions in 50. 00 ml of the following solution. 2 M K 3 P


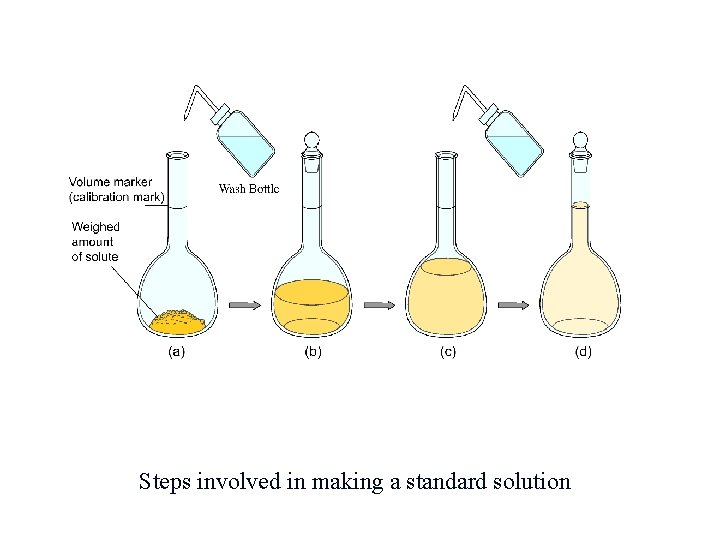
Standard Solution A standard solution is a solution whose concentration is accurately known. Standard solutions are made using a volumetric flask as follows: • mass the solute accurately and add it to the volumetric flask • add a small quantity of distilled HOH • dissolve the solute by gently swirling the flask • add more distilled HOH until the level of the solution reaches the mark on the neck • invert the capped volumetric 25 X to thoroughly mix the solution.

Steps involved in making a standard solution.

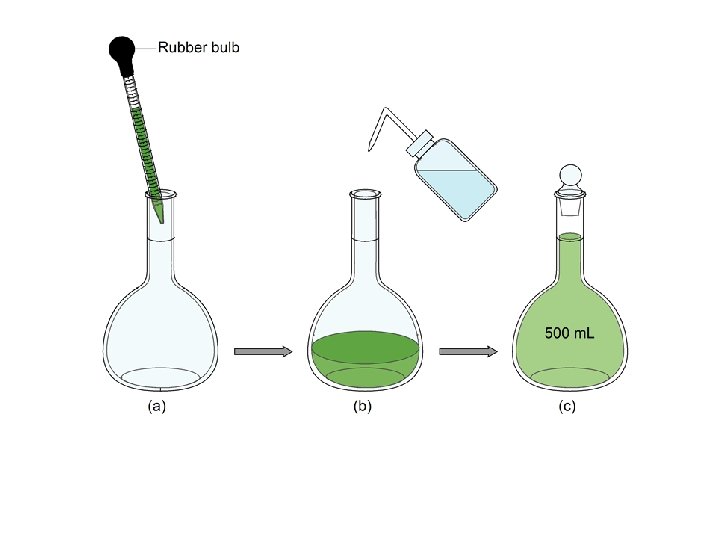
Steps to dilute a stock solution.

Common Terms of Solution Concentration Stock - routinely used solutions prepared in concentrated form. Concentrated - relatively large ratio of solute to solvent. (5. 0 M Na. Cl) Dilute - relatively small ratio of solute to solvent. (0. 01 M Na. Cl)



When diluting stock solutions, the moles of solute after dilution must equal the moles of solute before dilution. Stock solutions are diluted using either a measuring or a delivery pipet and a volumetric flask. What volume of 6 M sulfuric acid must be used to prepare 600 ml of a 1. 0 M H 2 SO 4 solution? use dilution formula What volume of 2 M sulfuric acid must be used to prepare 1 L of a. 5 M H 2 SO 4 solution?


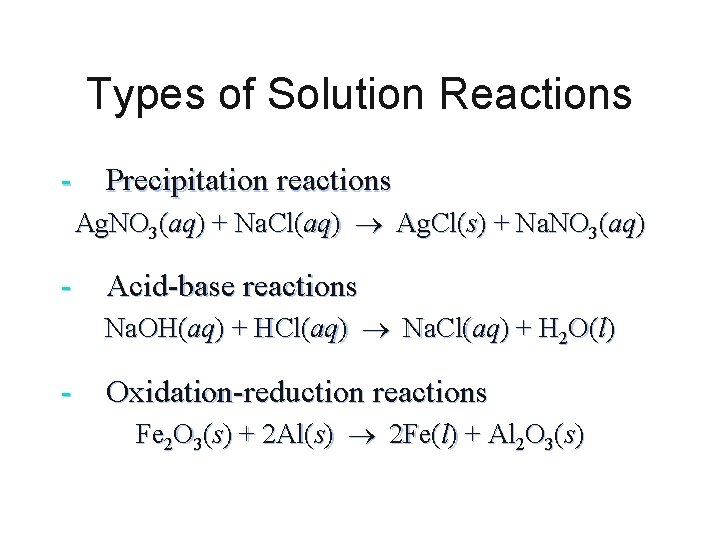
Types of Solution Reactions - Precipitation reactions Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) - Acid-base reactions Na. OH(aq) + HCl(aq) Na. Cl(aq) + H 2 O(l) - Oxidation-reduction reactions Fe 2 O 3(s) + 2 Al(s) 2 Fe(l) + Al 2 O 3(s)

1. Which of the following substances would you expect to be insoluble in water? Barium hydroxide Magnesium sulfate Silver chloride Calcium carbonate Ammonium acetate Sodium hydroxide Silver nitrate Hydrochloric acid Ammonium nitrate Lithium carbonate Barium sulfate Lead I Chloride Ammonium nitrate

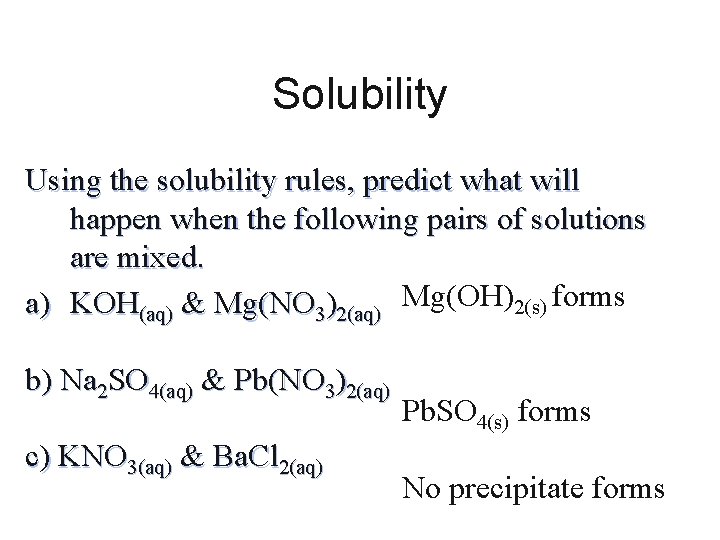
Solubility Using the solubility rules, predict what will happen when the following pairs of solutions are mixed. a) KOH(aq) & Mg(NO 3)2(aq) Mg(OH)2(s) forms b) Na 2 SO 4(aq) & Pb(NO 3)2(aq) c) KNO 3(aq) & Ba. Cl 2(aq) Pb. SO 4(s) forms No precipitate forms

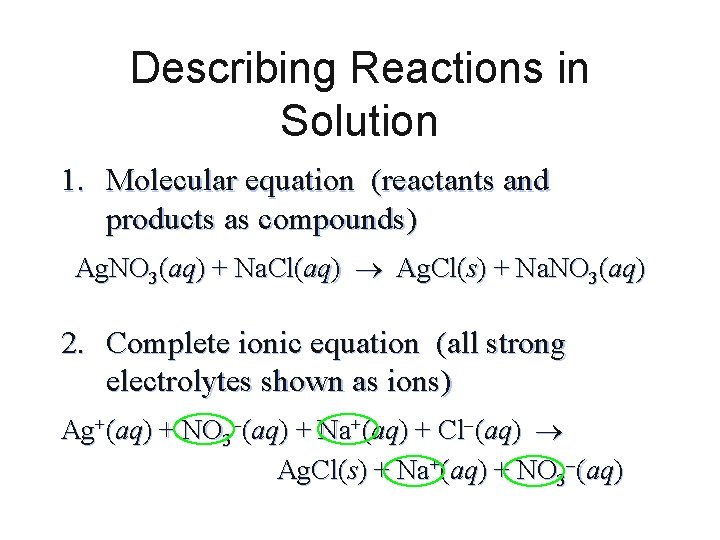
Describing Reactions in Solution 1. Molecular equation (reactants and products as compounds) Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) 2. Complete ionic equation (all strong electrolytes shown as ions) Ag+(aq) + NO 3 (aq) + Na+(aq) + Cl (aq) Ag. Cl(s) + Na+(aq) + NO 3 (aq)

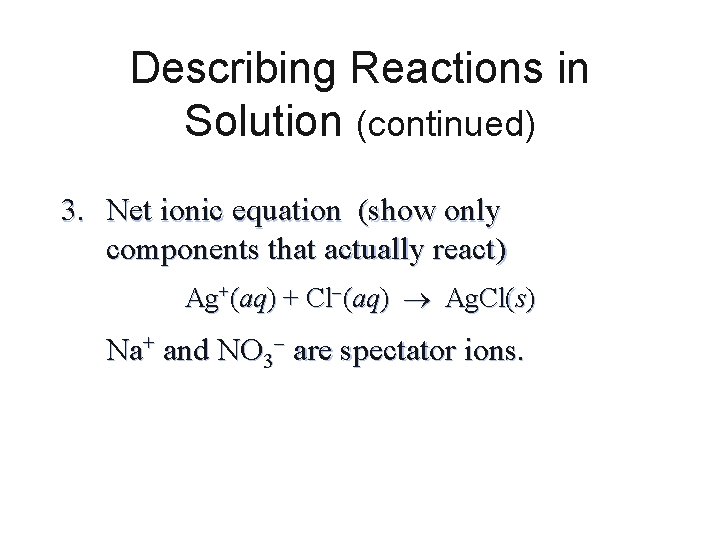
Describing Reactions in Solution (continued) 3. Net ionic equation (show only components that actually react) Ag+(aq) + Cl (aq) Ag. Cl(s) Na+ and NO 3 are spectator ions.

Write the balanced complete ionic and net ionic equations: Cu. SO 4(aq) + Li. Cl(aq) → P 108 q 28

Sodium Sulfate and Lead II Nitrate


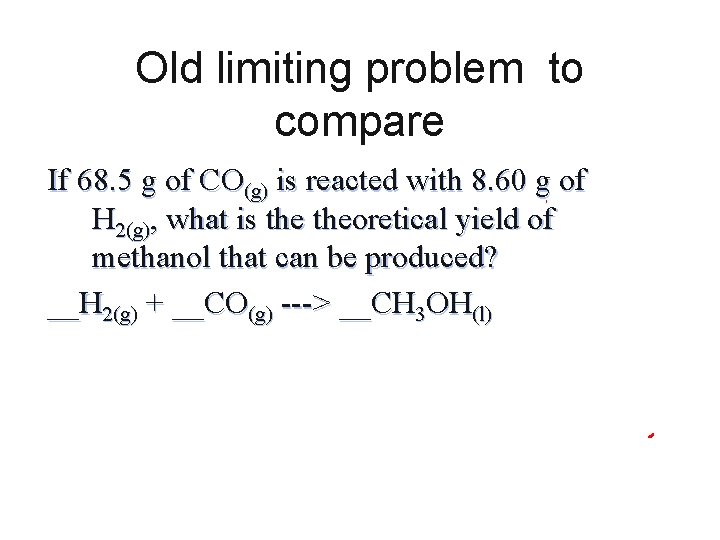
Old limiting problem to compare If 68. 5 g of CO(g) is reacted with 8. 60 g of H 2(g), what is theoretical yield of methanol that can be produced? __H 2(g) + __CO(g) ---> __CH 3 OH(l)

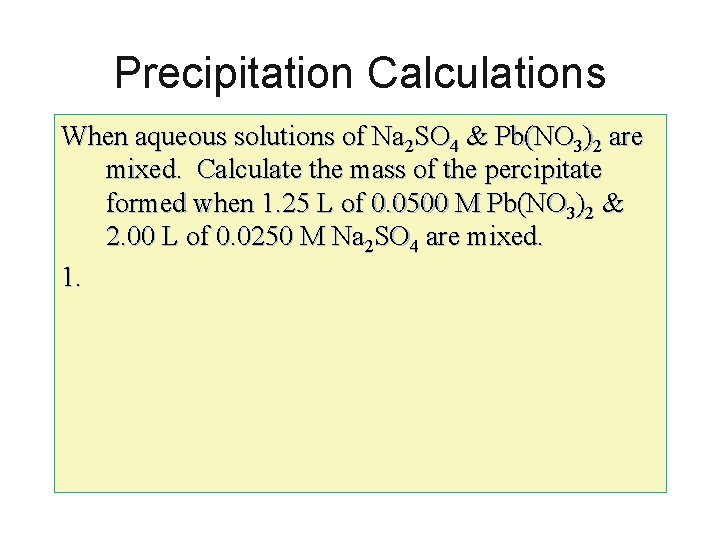
Precipitation Calculations When aqueous solutions of Na 2 SO 4 & Pb(NO 3)2 are mixed. Calculate the mass of the percipitate formed when 1. 25 L of 0. 0500 M Pb(NO 3)2 & 2. 00 L of 0. 0250 M Na 2 SO 4 are mixed. 1.

Now it’s the same as limiting you already know.

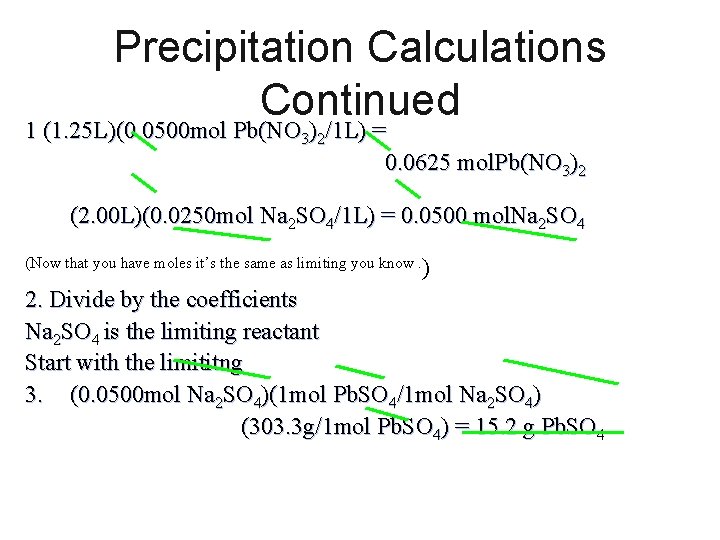
Precipitation Calculations Continued 1 (1. 25 L)(0. 0500 mol Pb(NO 3)2/1 L) = 0. 0625 mol. Pb(NO 3)2 (2. 00 L)(0. 0250 mol Na 2 SO 4/1 L) = 0. 0500 mol. Na 2 SO 4 (Now that you have moles it’s the same as limiting you know. ) 2. Divide by the coefficients Na 2 SO 4 is the limiting reactant Start with the limititng 3. (0. 0500 mol Na 2 SO 4)(1 mol Pb. SO 4/1 mol Na 2 SO 4) (303. 3 g/1 mol Pb. SO 4) = 15. 2 g Pb. SO 4

PRACTICE What mass of precipitate should result when 0. 550 L of 0. 500 M aluminum nitrate solution is mixed with 0. 240 L of 1. 50 M sodium hydroxide solution?


What mass of precipitate should result when 0. 350 L of 0. 200 M aluminum nitrate solution is mixed with 0. 540 L of. 50 M sodium hydroxide solution? What is the net ionic equation? P 108 29 -32


Mixed Limiting A. 750 g sample of Iron II Chloride is dissolved in water and a mixed with 22. 40 ml of. 515 M Ag. NO 3. Calculate the grams of precipitate.


Practice precipitation What mass of Fe(OH)3 is produced when 35. ml of a. 250 M Fe(NO 3)3 solution is mixed with 55 ml of a 0. 180 M KOH? Green book p 95


In the experiment, a student is given 2. 94 g of a mixture containing anhydrous Mg. Cl 2 and KNO 3. To determine the percentage by mass of Mg. Cl 2 in the mixture, the student uses excess Ag. NO 3(aq) to precipitate the chloride ion as Ag. Cl(s). The student determines the mass of the Ag. Cl precipitate to be 5. 48 g. On the basis of this information, calculate The number of moles of Mg. Cl 2 in the original mixture.




Green book review p 106 # 44 practice gravimetric analysis A 2. 10 g sample of silver alloy was dissolved and the precipitated as Ag. Br. After washing and drying the silver bromide weighed 2. 00 g. Calculate the percentage silver in the alloy.





What volume of 0. 415 M silver nitrate will be required to precipitate as silver bromide all the bromide ion in 35. 0 m. L of 0. 128 M calcium bromide?

 Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Are kc and kp equal
Are kc and kp equal Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions Tyoes of chemical reactions
Tyoes of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Combustion chemical reaction definition
Combustion chemical reaction definition 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Types of reactions
Types of reactions Three types of chemical reactions
Three types of chemical reactions Combustion chemical reaction
Combustion chemical reaction How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Synthesis reaction
Synthesis reaction What are active metals
What are active metals Redox reaction example
Redox reaction example Unit 5 chemical reactions answers
Unit 5 chemical reactions answers How to find moles from molarity
How to find moles from molarity Solution stoichiometry
Solution stoichiometry Solution stoichiometry
Solution stoichiometry Solution stoichiometry
Solution stoichiometry Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry In a chemical reaction, stoichiometry refers to
In a chemical reaction, stoichiometry refers to Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Chapter 11 stoichiometry answer key
Chapter 11 stoichiometry answer key Reschual
Reschual Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Thermodynamics chapter 2
Thermodynamics chapter 2 Reactions that produce gas
Reactions that produce gas Half cell reaction
Half cell reaction Empirical formula pogil
Empirical formula pogil Chapter 7 chemical formulas and chemical compounds
Chapter 7 chemical formulas and chemical compounds Stoichiometry island diagram
Stoichiometry island diagram I intro
I intro Predicting products of chemical reactions
Predicting products of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions Activity series of metals
Activity series of metals Unit 11 chemical reactions
Unit 11 chemical reactions