TYPES OF CHEMICAL REACTIONS Precipitation Reactions Acids Bases
TYPES OF CHEMICAL REACTIONS • • • Precipitation Reactions Acids Bases Neutralization Combustion Oxidation-Reduction 1
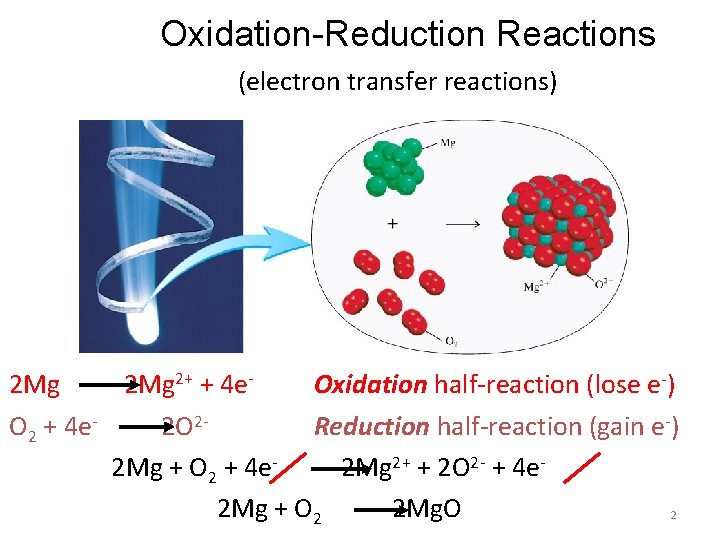
Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg + O 2 2 Mg. O 2
3
Types of Chemical Reactions Oxidation Reduction Reactions • Electrons are transferred • Oxidation: Loss of Electrons • Reduction: Acceptance of Electrons
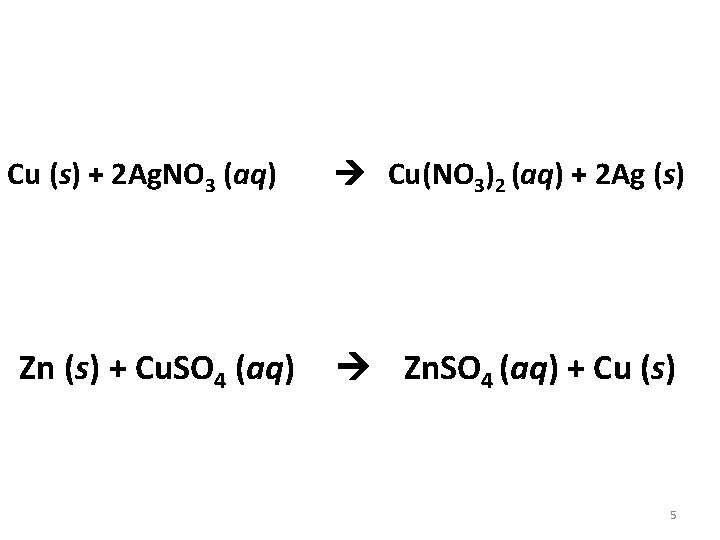
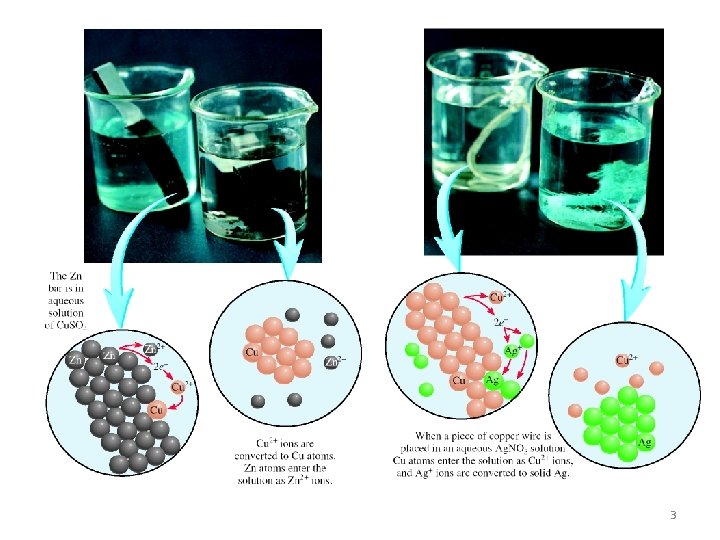
Cu (s) + 2 Ag. NO 3 (aq) Zn (s) + Cu. SO 4 (aq) Cu(NO 3)2 (aq) + 2 Ag (s) Zn. SO 4 (aq) + Cu (s) 5
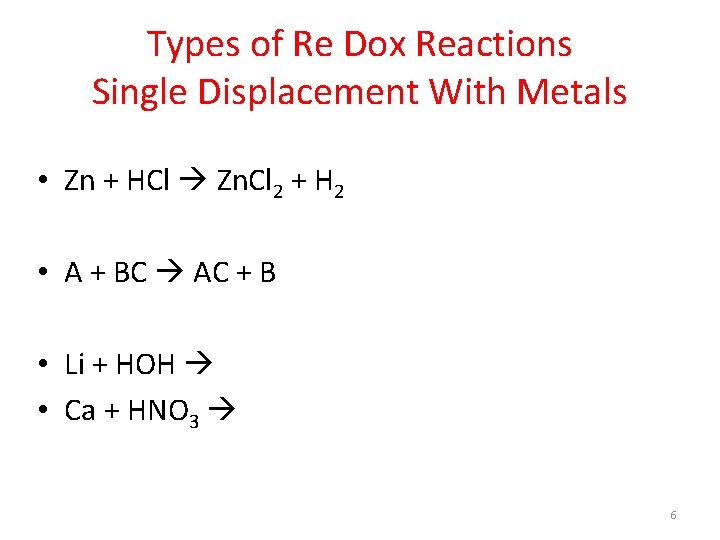
Types of Re Dox Reactions Single Displacement With Metals • Zn + HCl Zn. Cl 2 + H 2 • A + BC AC + B • Li + HOH • Ca + HNO 3 6
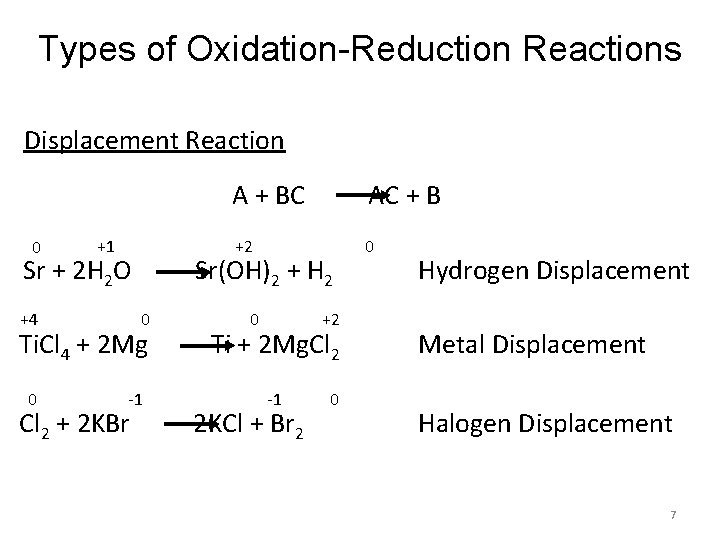
Types of Oxidation-Reduction Reactions Displacement Reaction 0 +1 Sr + 2 H 2 O +4 0 -1 Cl 2 + 2 KBr AC + B +2 0 Sr(OH)2 + H 2 Ti. Cl 4 + 2 Mg 0 A + BC 0 +2 Ti + 2 Mg. Cl 2 -1 2 KCl + Br 2 0 Hydrogen Displacement Metal Displacement Halogen Displacement 7
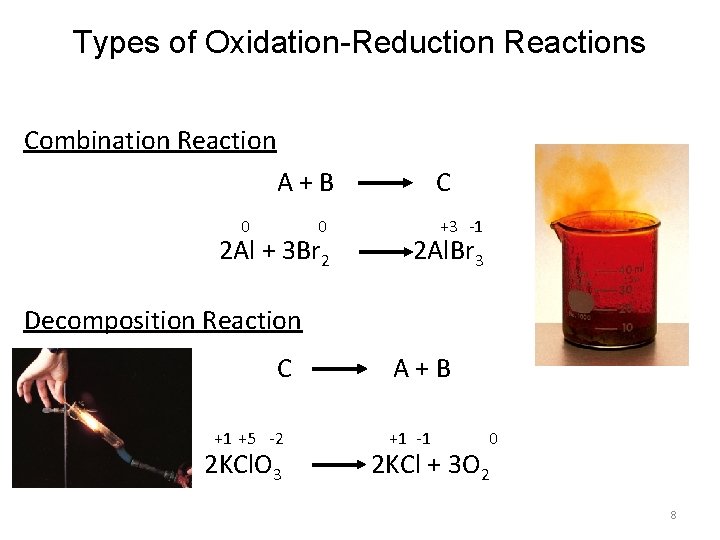
Types of Oxidation-Reduction Reactions Combination Reaction A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2 8
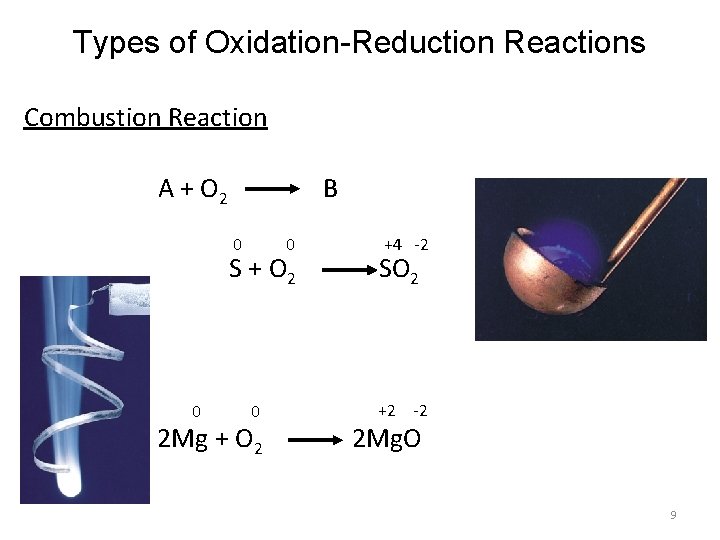
Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 B 0 0 S + O 2 0 0 2 Mg + O 2 +4 -2 SO 2 +2 -2 2 Mg. O 9
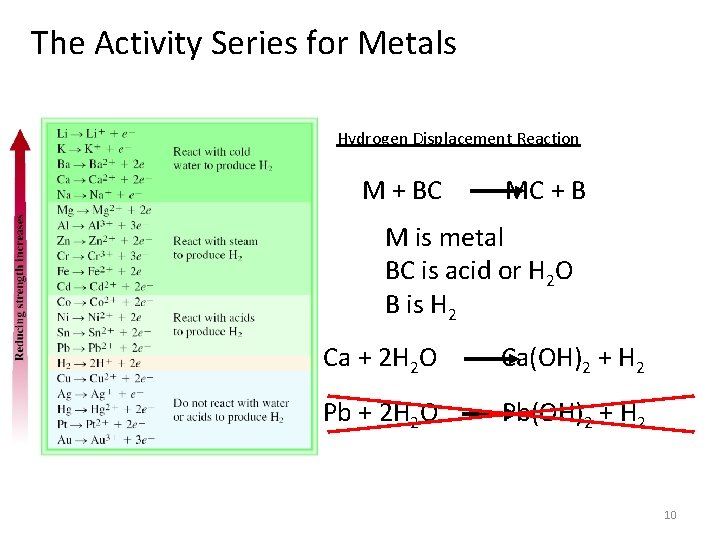
The Activity Series for Metals Hydrogen Displacement Reaction M + BC MC + B M is metal BC is acid or H 2 O B is H 2 Ca + 2 H 2 O Ca(OH)2 + H 2 Pb + 2 H 2 O Pb(OH)2 + H 2 10
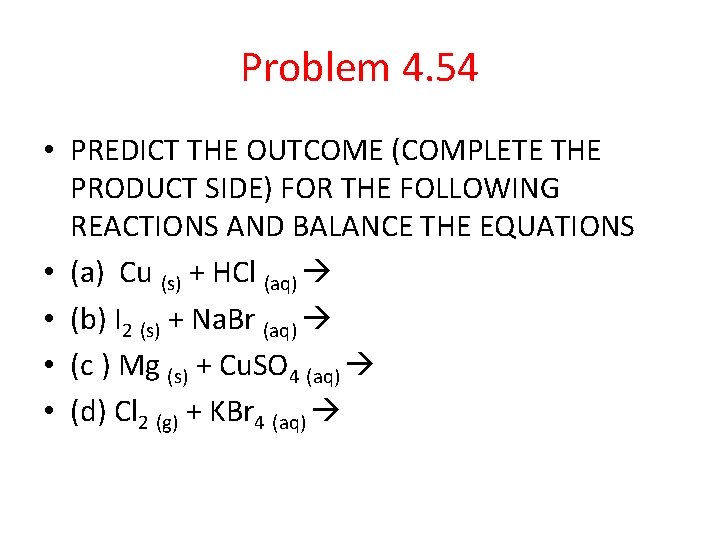
Problem 4. 54 • PREDICT THE OUTCOME (COMPLETE THE PRODUCT SIDE) FOR THE FOLLOWING REACTIONS AND BALANCE THE EQUATIONS • (a) Cu (s) + HCl (aq) • (b) I 2 (s) + Na. Br (aq) • (c ) Mg (s) + Cu. SO 4 (aq) • (d) Cl 2 (g) + KBr 4 (aq)
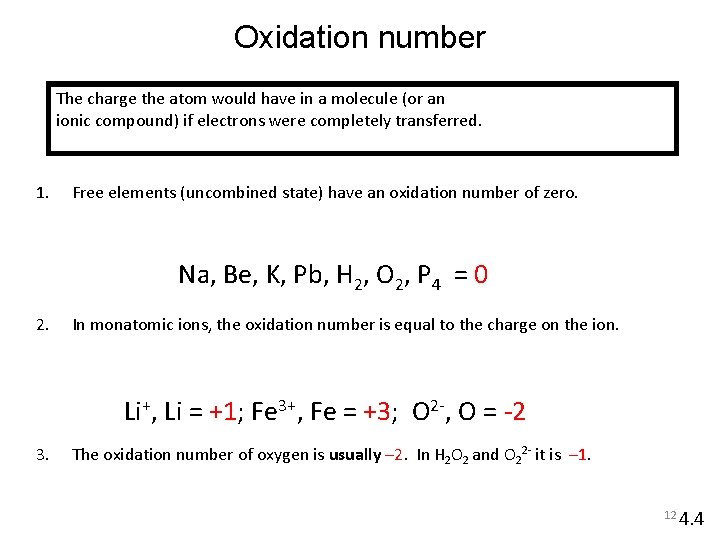
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1. 12 4. 4
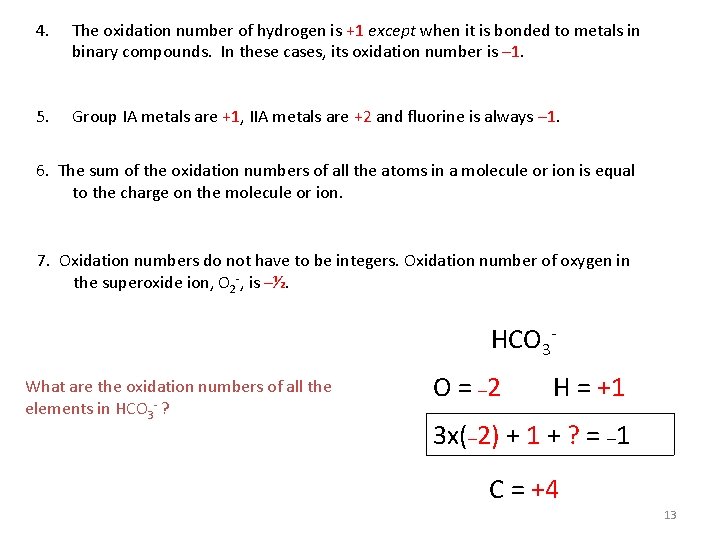
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O 2 -, is –½. HCO 3 What are the oxidation numbers of all the elements in HCO 3 - ? O = – 2 H = +1 3 x(– 2) + 1 + ? = – 1 C = +4 13
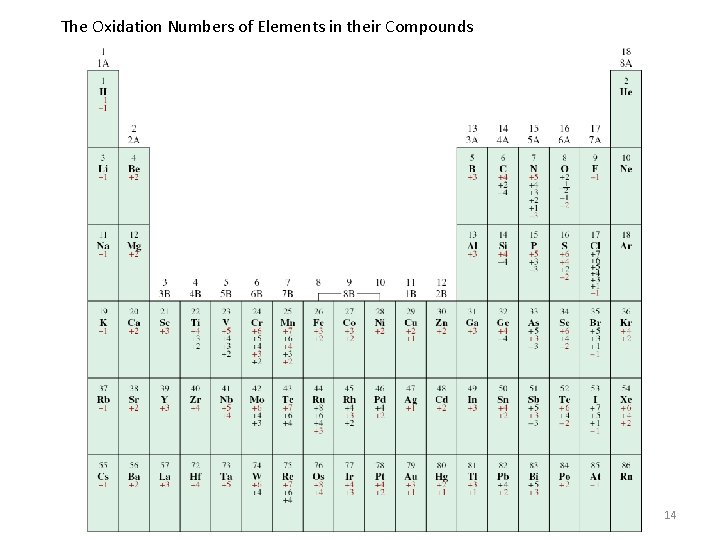
The Oxidation Numbers of Elements in their Compounds 14
- Slides: 14