Types of Chemical Reactions Precipitation part 2 Predicting





- Slides: 23

Types of Chemical Reactions Precipitation part 2 Predicting the Formation of a Precipitate

Objective � I can use the solubility rules to predict the precipitate formed in precipitation (double replacement) reactions

Precipitation reactions � Our world is water-based. � More than 70% of the Earth’s surface is covered by water, � About 66% of the human body is water. � Many important chemical reactions take place with compounds dissolved water – that is, in aqueous solution. � definitions to know: � soluble: Able to be dissolved in water � insoluble: Not able to be dissolved in water

Which of these means soluble? A. B. like sugar does in a cup of hot tea like sand does at the beach

Which of these is insoluble? A. B. you stir something into water and it seems to disappear. you stir something into water and it all just settles on the bottom

Which of the following is a member of the alkali metal group? A. B. C. D. E. AB C DE

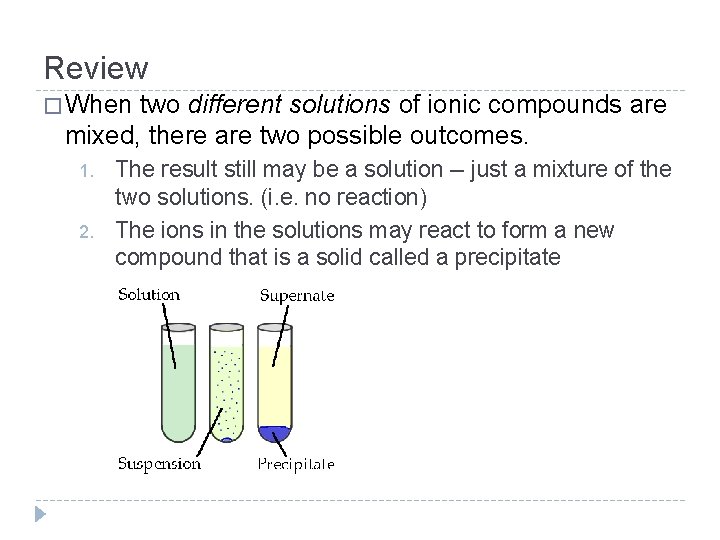
Review � When two different solutions of ionic compounds are mixed, there are two possible outcomes. 1. 2. The result still may be a solution -- just a mixture of the two solutions. (i. e. no reaction) The ions in the solutions may react to form a new compound that is a solid called a precipitate

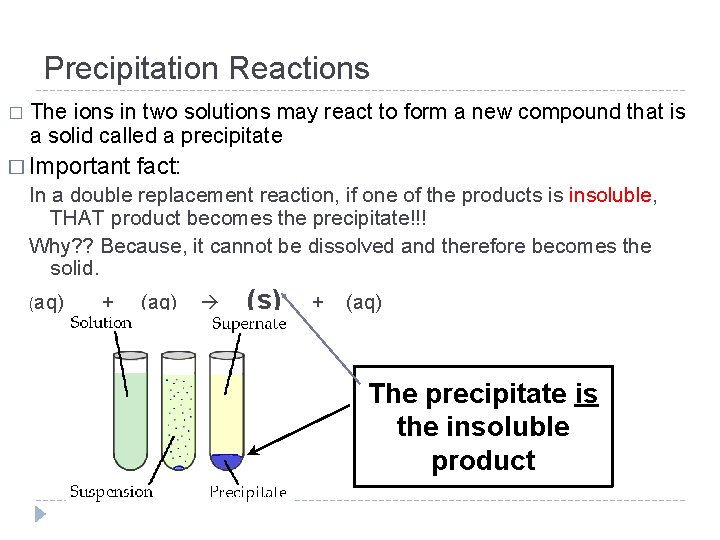
Precipitation Reactions � The ions in two solutions may react to form a new compound that is a solid called a precipitate � Important fact: In a double replacement reaction, if one of the products is insoluble, THAT product becomes the precipitate!!! Why? ? Because, it cannot be dissolved and therefore becomes the solid. (aq) + (aq) (s) + (aq) The precipitate is the insoluble product

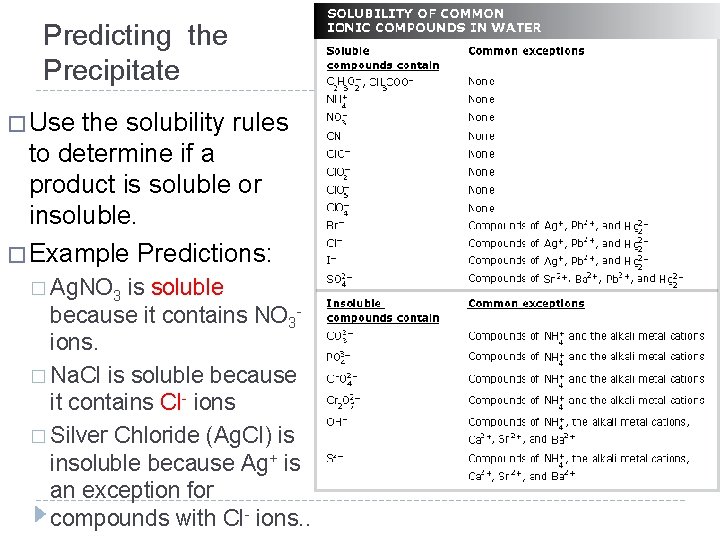
Predicting the Precipitate � Use the solubility rules to determine if a product is soluble or insoluble. � Example Predictions: � Ag. NO 3 is soluble because it contains NO 3 ions. � Na. Cl is soluble because it contains Cl- ions � Silver Chloride (Ag. Cl) is insoluble because Ag+ is an exception for compounds with Cl- ions. .

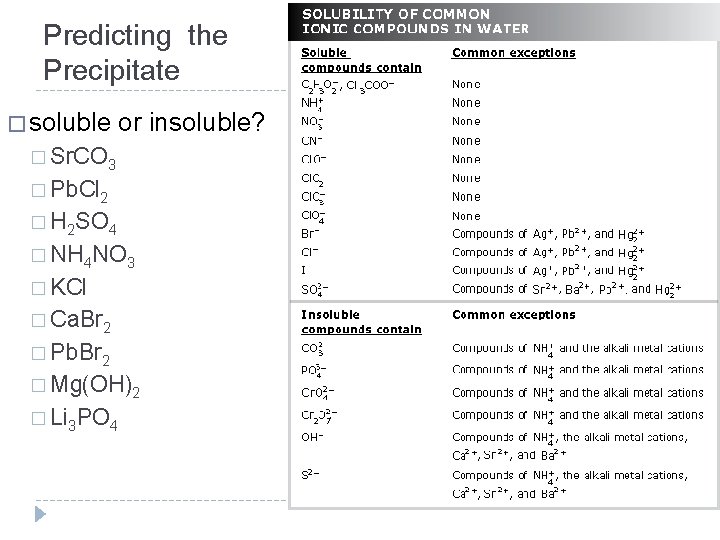
Predicting the Precipitate � soluble or insoluble? � Sr. CO 3 � Pb. Cl 2 � H 2 SO 4 � NH 4 NO 3 � KCl � Ca. Br 2 � Pb. Br 2 � Mg(OH)2 � Li 3 PO 4

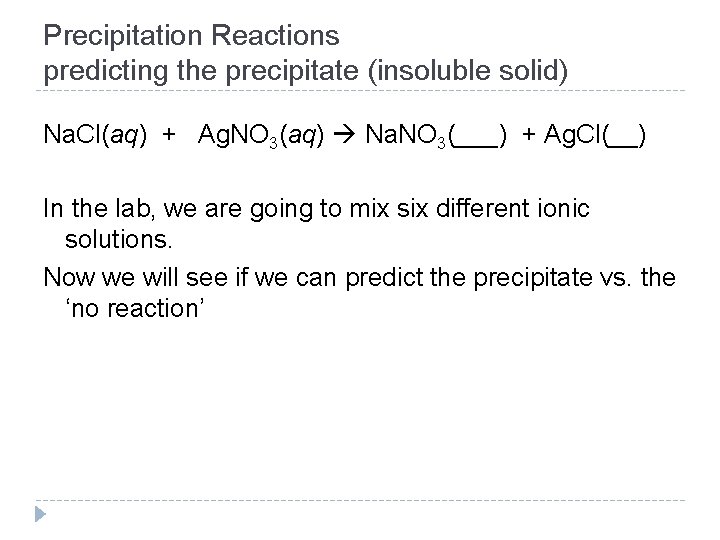
Precipitation Reactions predicting the precipitate (insoluble solid) Na. Cl(aq) + Ag. NO 3(aq) Na. NO 3(___) + Ag. Cl(__) In the lab, we are going to mix six different ionic solutions. Now we will see if we can predict the precipitate vs. the ‘no reaction’

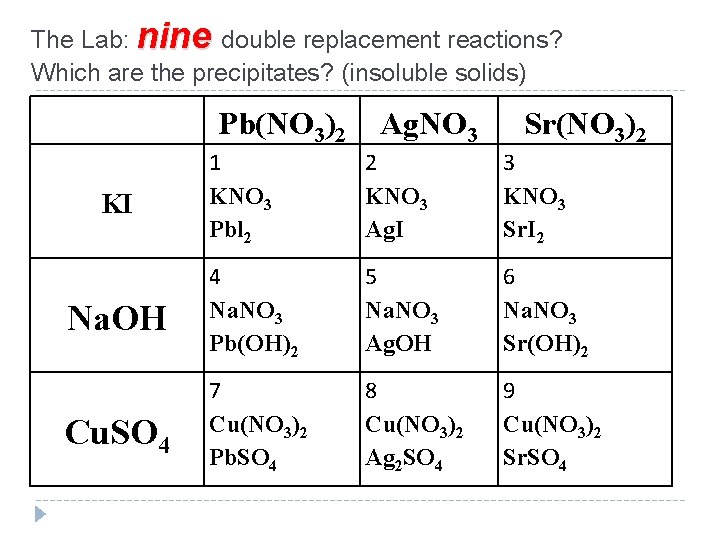
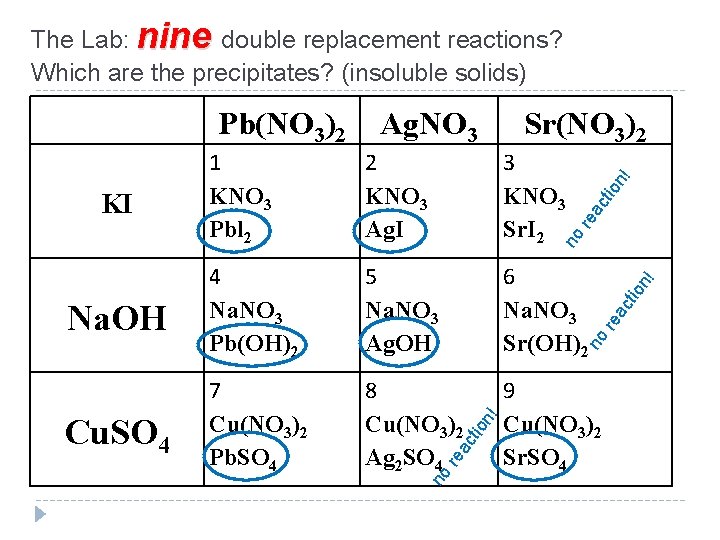
The Lab: nine double replacement reactions? Which are the precipitates? (insoluble solids) Pb(NO 3)2 Ag. NO 3 Sr(NO 3)2 1 KNO 3 Pbl 2 2 KNO 3 Ag. I 3 KNO 3 Sr. I 2 Na. OH 4 Na. NO 3 Pb(OH)2 5 Na. NO 3 Ag. OH 6 Na. NO 3 Sr(OH)2 Cu. SO 4 7 Cu(NO 3)2 Pb. SO 4 8 Cu(NO 3)2 Ag 2 SO 4 9 Cu(NO 3)2 Sr. SO 4 KI

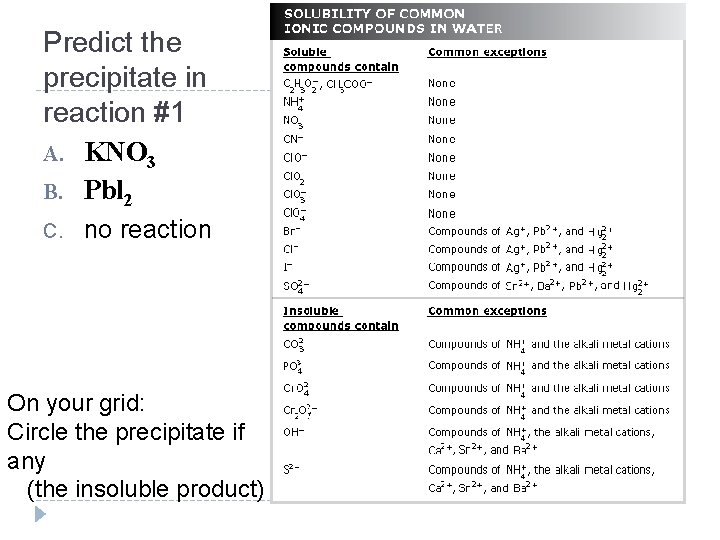
Predict the precipitate in reaction #1 A. KNO 3 B. Pbl 2 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

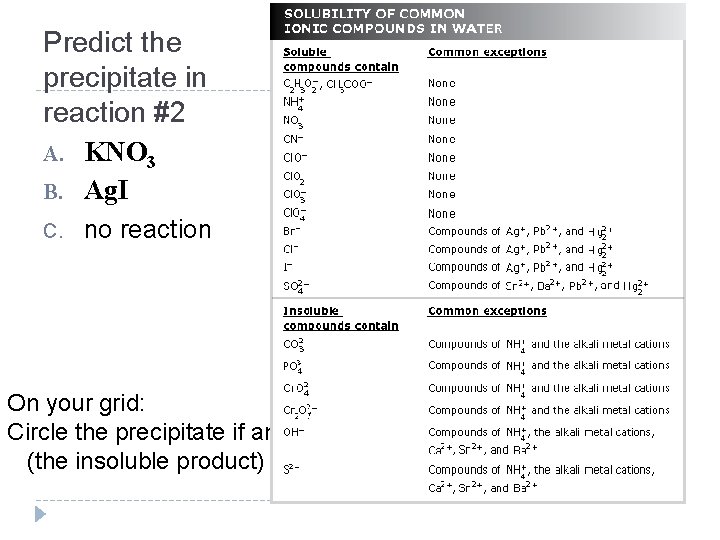
Predict the precipitate in reaction #2 A. KNO 3 B. Ag. I C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

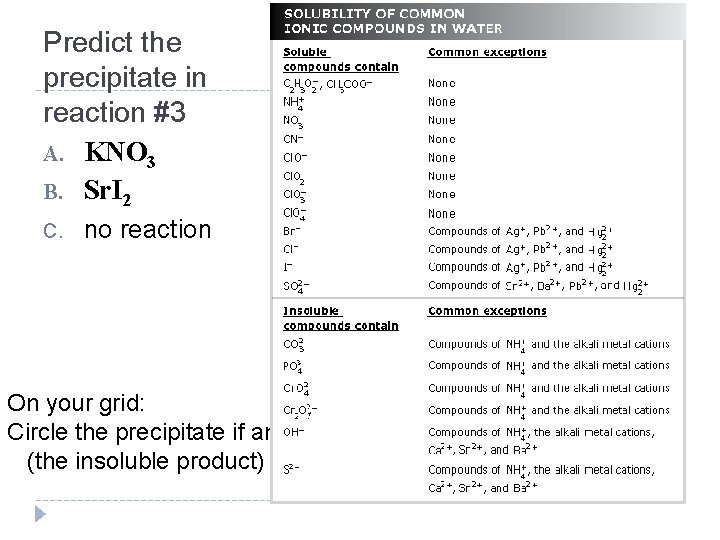
Predict the precipitate in reaction #3 A. KNO 3 B. Sr. I 2 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

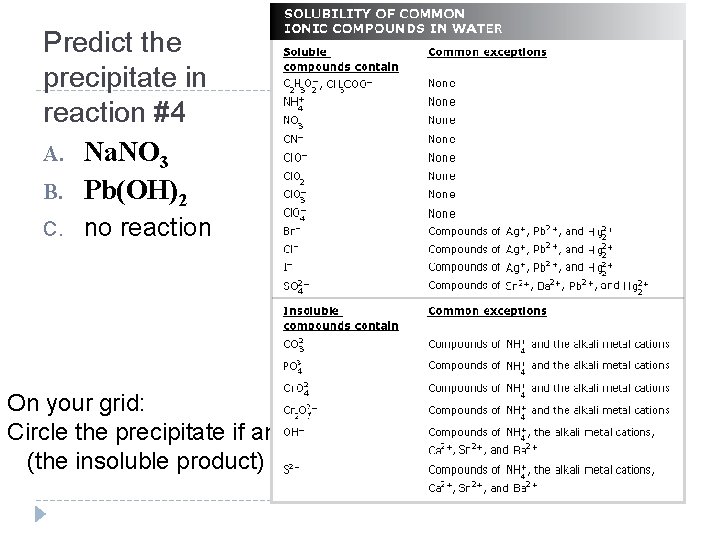
Predict the precipitate in reaction #4 A. Na. NO 3 B. Pb(OH)2 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

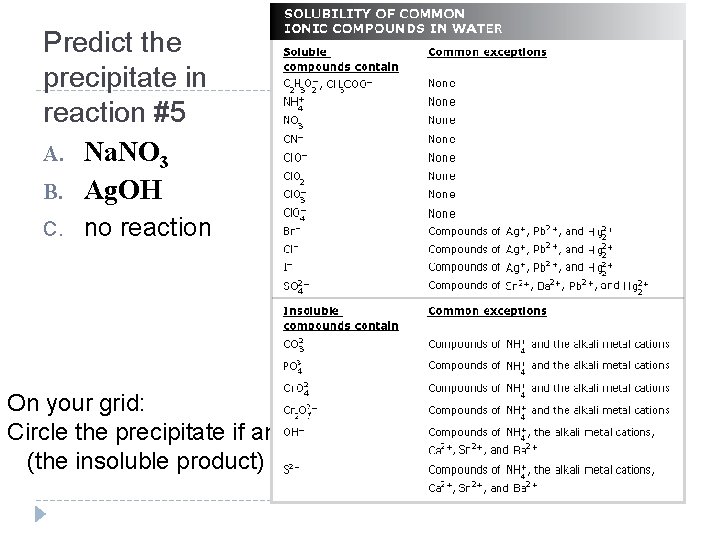
Predict the precipitate in reaction #5 A. Na. NO 3 B. Ag. OH C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

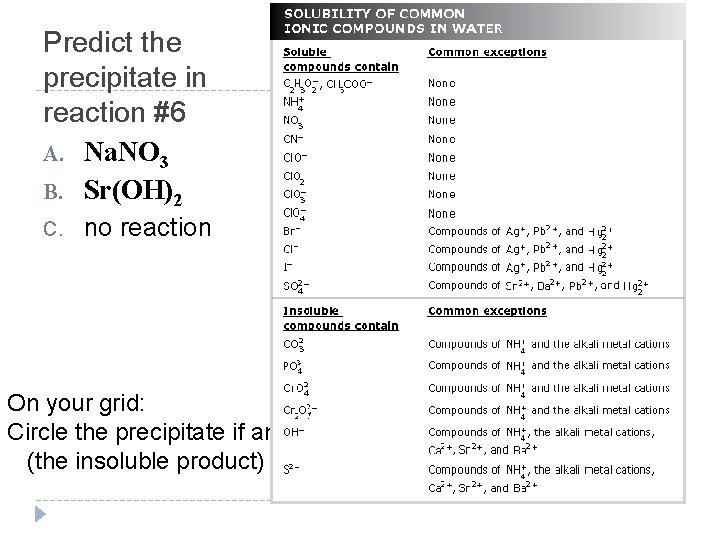
Predict the precipitate in reaction #6 A. Na. NO 3 B. Sr(OH)2 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

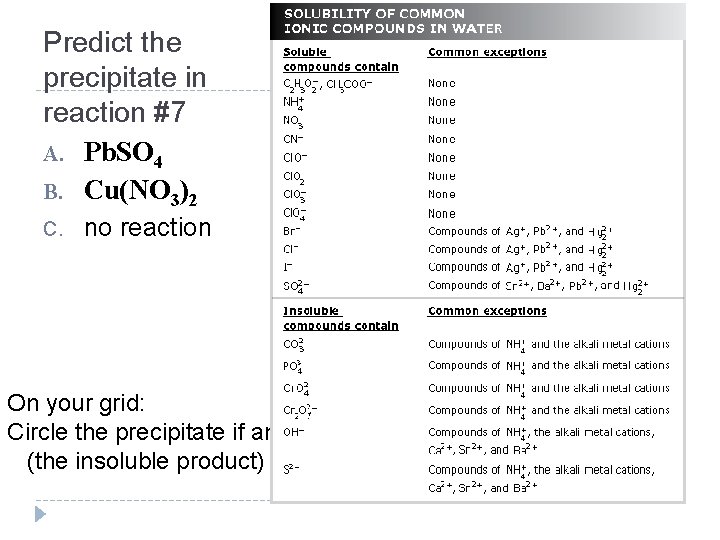
Predict the precipitate in reaction #7 A. Pb. SO 4 B. Cu(NO 3)2 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

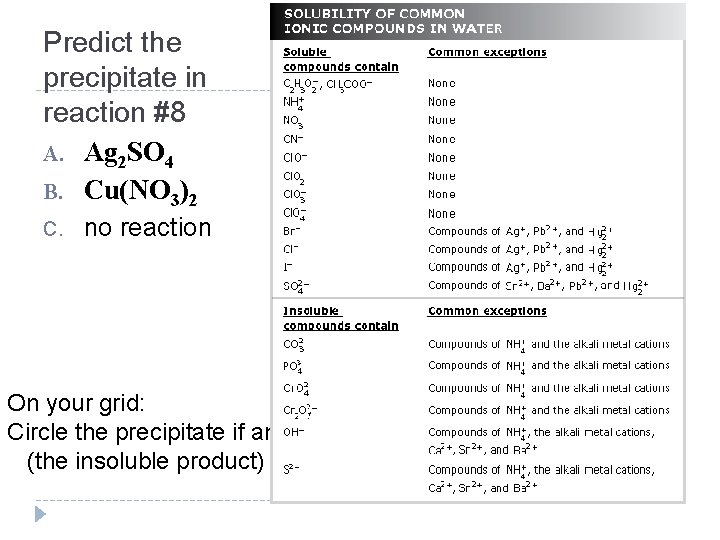
Predict the precipitate in reaction #8 A. Ag 2 SO 4 B. Cu(NO 3)2 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

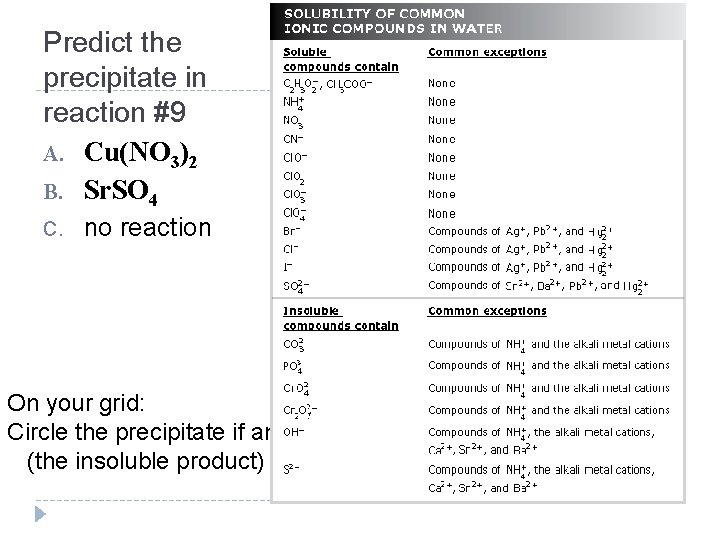
Predict the precipitate in reaction #9 A. Cu(NO 3)2 B. Sr. SO 4 C. no reaction On your grid: Circle the precipitate if any (the insoluble product)

The Lab: nine double replacement reactions? Which are the precipitates? (insoluble solids) Pb(NO 3)2 Ag. NO 3 Sr(NO 3)2 2 KNO 3 Ag. I 3 KNO 3 Sr. I 2 Na. OH 4 Na. NO 3 Pb(OH)2 5 Na. NO 3 Ag. OH 6 Na. NO 3 Sr(OH)2 Cu. SO 4 7 Cu(NO 3)2 Pb. SO 4 8 Cu(NO 3)2 Ag 2 SO 4 9 Cu(NO 3)2 Sr. SO 4 ac tio re no re a cti on ! no on ! cti re a no KI n! 1 KNO 3 Pbl 2

Objective � I can use the solubility rules to predict the precipitate formed in precipitation (double replacement) reactions