Types of Chemical Reactions Power Point 6 1
- Slides: 9

Types of Chemical Reactions Power. Point 6. 1

Six Common Types of Reactions to Know 1. Synthesis (Combination) Reactions 2. Decomposition Reactions 3. Single Replacement 4. Double Replacement 5. Neutralization (Acid-Base) Reactions 6. Combustion Reactions

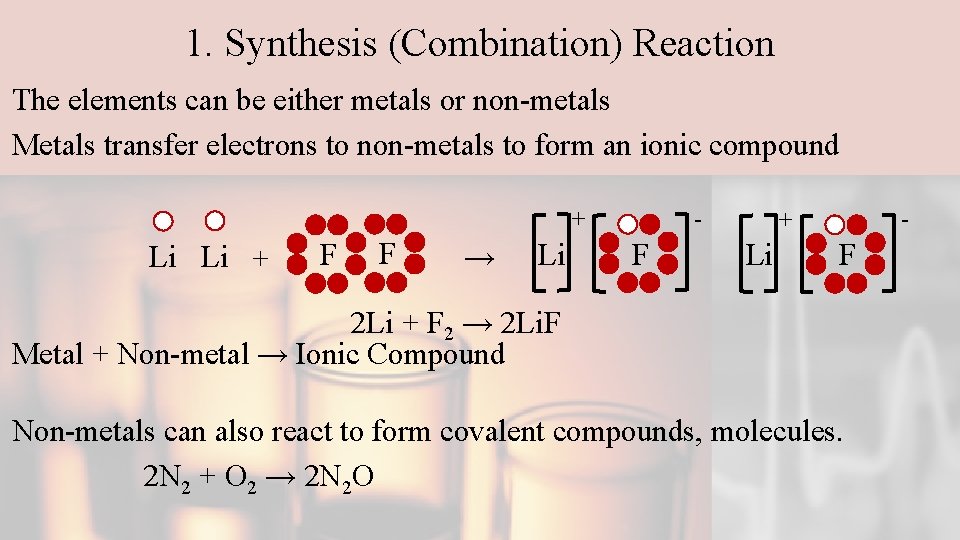
1. Synthesis (Combination) Reaction The elements can be either metals or non-metals Metals transfer electrons to non-metals to form an ionic compound - + Li Li + F F → Li F - + Li F 2 Li + F 2 → 2 Li. F Metal + Non-metal → Ionic Compound Non-metals can also react to form covalent compounds, molecules. 2 N 2 + O 2 → 2 N 2 O

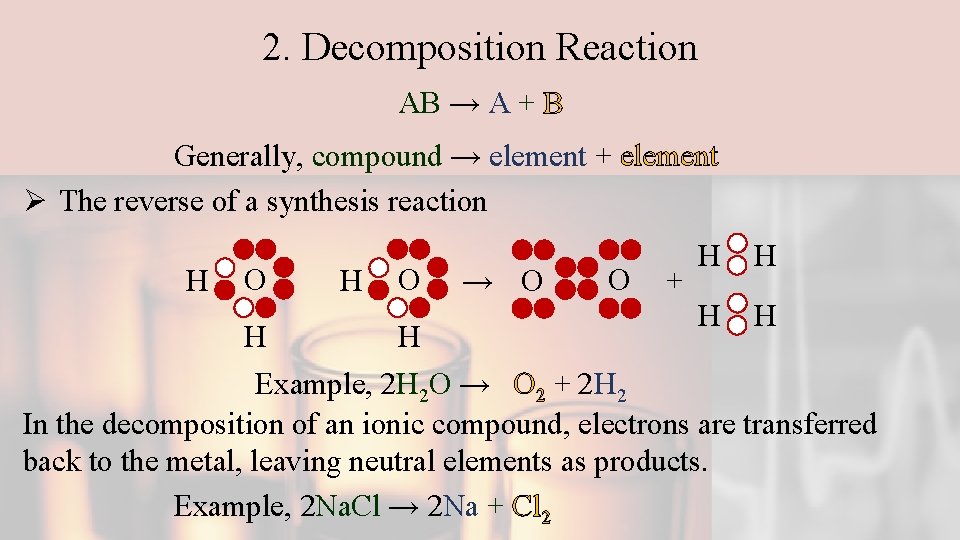
2. Decomposition Reaction AB → A + B Generally, compound → element + element Ø The reverse of a synthesis reaction H O H → O O + H H Example, 2 H 2 O → O 2 + 2 H 2 In the decomposition of an ionic compound, electrons are transferred back to the metal, leaving neutral elements as products. Example, 2 Na. Cl → 2 Na + Cl 2

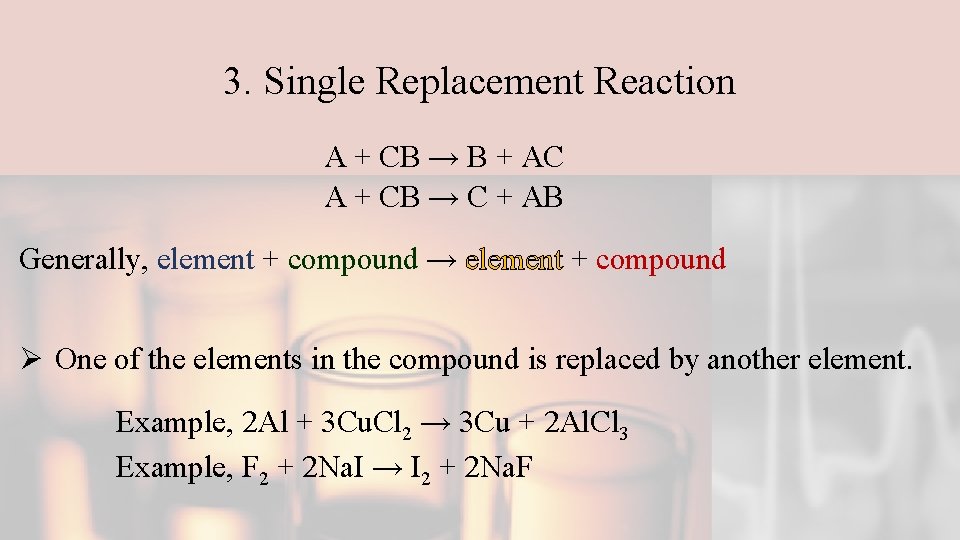
3. Single Replacement Reaction A + CB → B + AC A + CB → C + AB Generally, element + compound → element + compound Ø One of the elements in the compound is replaced by another element. Example, 2 Al + 3 Cu. Cl 2 → 3 Cu + 2 Al. Cl 3 Example, F 2 + 2 Na. I → I 2 + 2 Na. F

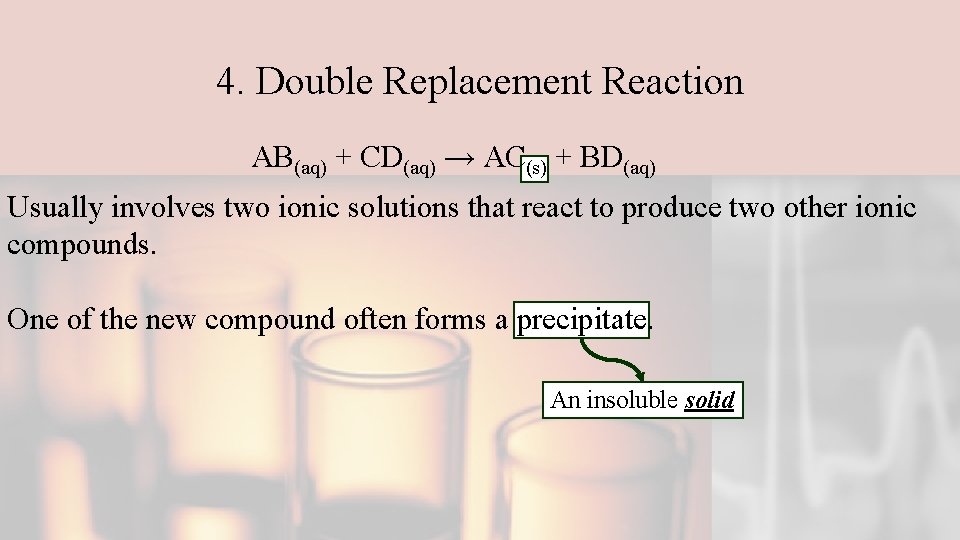
4. Double Replacement Reaction AB(aq) + CD(aq) → AC(s) + BD(aq) Usually involves two ionic solutions that react to produce two other ionic compounds. One of the new compound often forms a precipitate. An insoluble solid

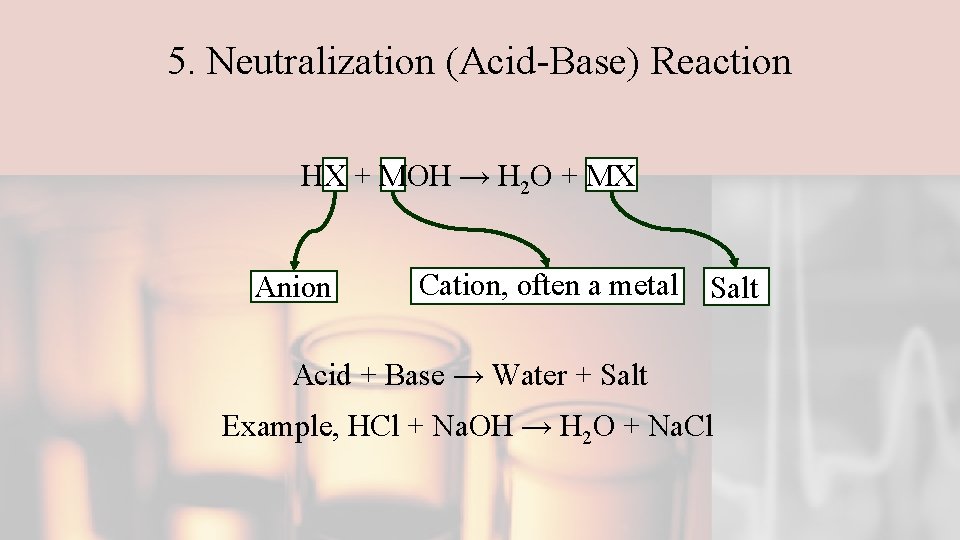
5. Neutralization (Acid-Base) Reaction HX + MOH → H 2 O + MX Anion Cation, often a metal Salt Acid + Base → Water + Salt Example, HCl + Na. OH → H 2 O + Na. Cl

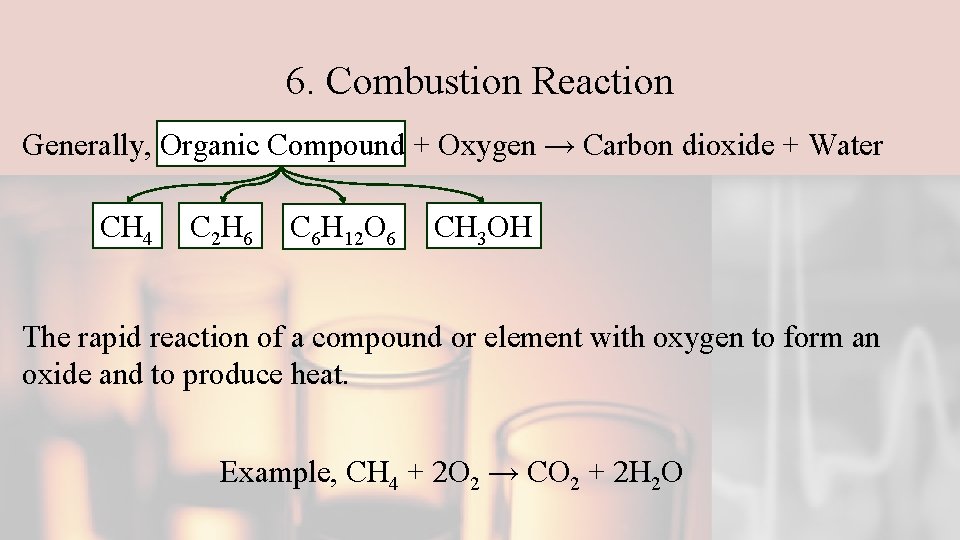
6. Combustion Reaction Generally, Organic Compound + Oxygen → Carbon dioxide + Water CH 4 C 2 H 6 C 6 H 12 O 6 CH 3 OH The rapid reaction of a compound or element with oxygen to form an oxide and to produce heat. Example, CH 4 + 2 O 2 → CO 2 + 2 H 2 O

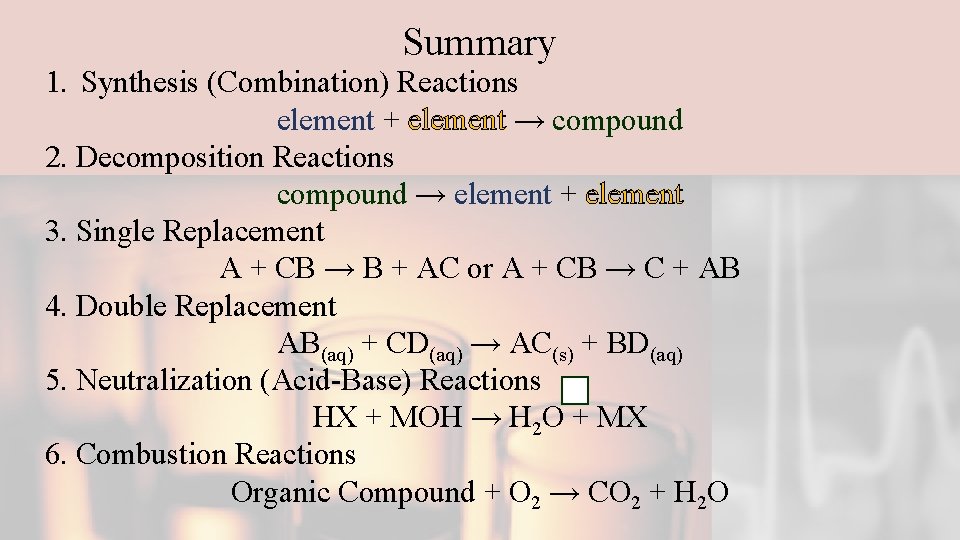
Summary 1. Synthesis (Combination) Reactions element + element → compound 2. Decomposition Reactions compound → element + element 3. Single Replacement A + CB → B + AC or A + CB → C + AB 4. Double Replacement AB(aq) + CD(aq) → AC(s) + BD(aq) 5. Neutralization (Acid-Base) Reactions HX + MOH → H 2 O + MX 6. Combustion Reactions Organic Compound + O 2 → CO 2 + H 2 O