Types of Chemical Reactions Learning Target To be

Types of Chemical Reactions Learning Target: To be able to identify the different type of reactions when looking at skeletal equations

Types of Reactions: �There 1. 2. 3. 4. 5. are 5 types of Reactions: Synthesis Decomposition Single-replacement Double-replacement Combustion
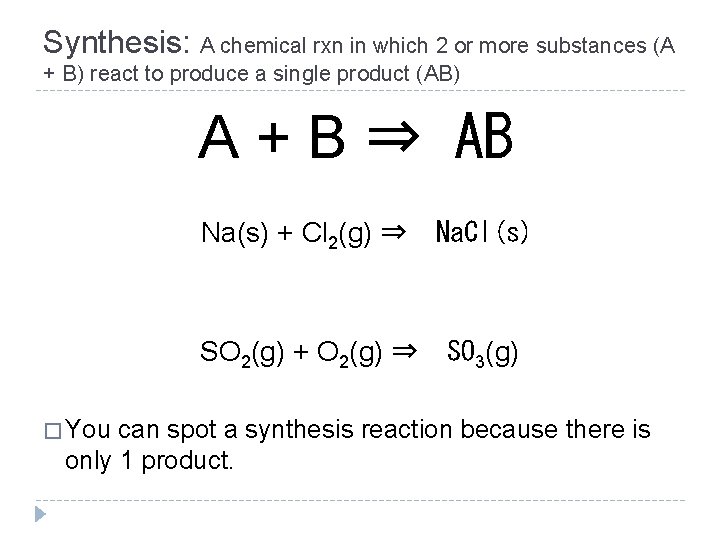
Synthesis: A chemical rxn in which 2 or more substances (A + B) react to produce a single product (AB) A + B ⇒ AB � You Na(s) + Cl 2(g) ⇒ Na. Cl(s) SO 2(g) + O 2(g) ⇒ SO 3(g) can spot a synthesis reaction because there is only 1 product.
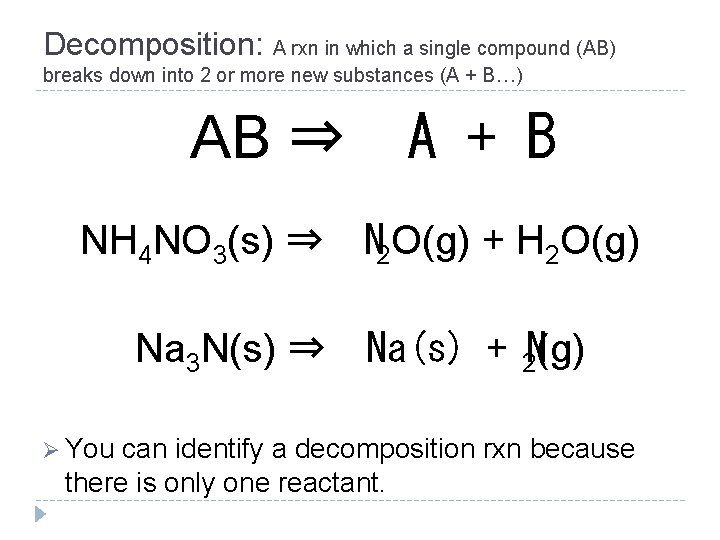
Decomposition: A rxn in which a single compound (AB) breaks down into 2 or more new substances (A + B…) AB ⇒ A + B NH 4 NO 3(s) ⇒ Na 3 N(s) ⇒ Ø You N 2 O(g) + H 2 O(g) Na(s) + 2 N(g) can identify a decomposition rxn because there is only one reactant.
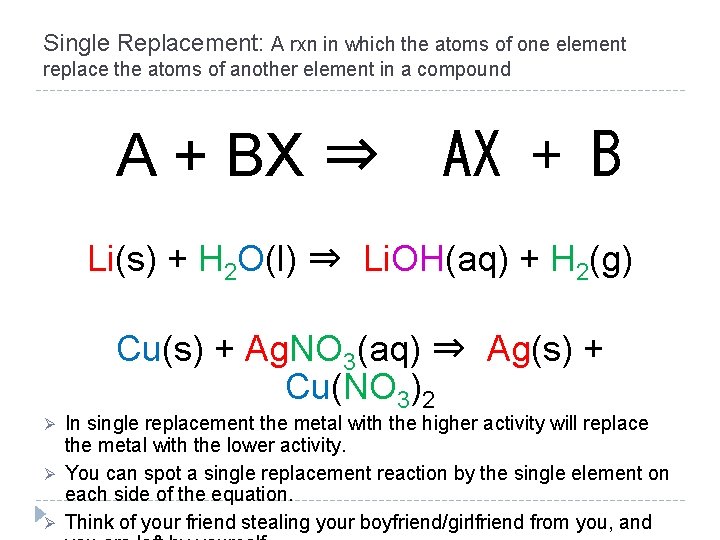
Single Replacement: A rxn in which the atoms of one element replace the atoms of another element in a compound A + BX ⇒ AX + B Li(s) + H 2 O(l) ⇒ Li. OH(aq) + H 2(g) Cu(s) + Ag. NO 3(aq) ⇒ Ag(s) + Cu(NO 3)2 Ø Ø Ø In single replacement the metal with the higher activity will replace the metal with the lower activity. You can spot a single replacement reaction by the single element on each side of the equation. Think of your friend stealing your boyfriend/girlfriend from you, and
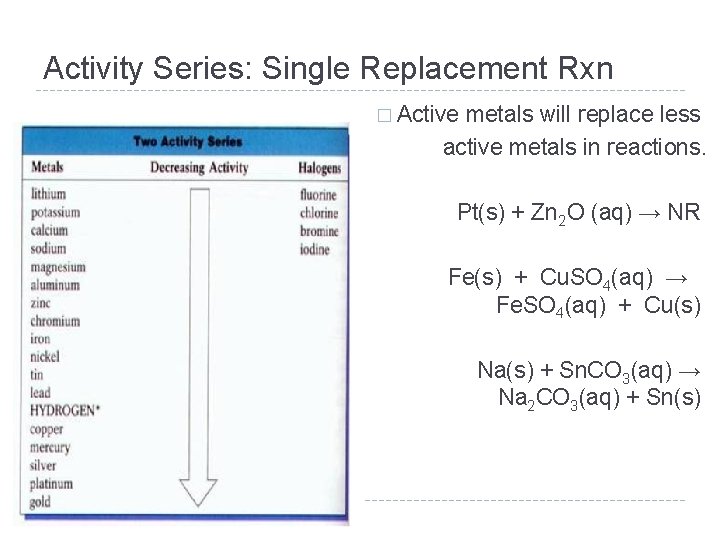
Activity Series: Single Replacement Rxn � Active metals will replace less active metals in reactions. Pt(s) + Zn 2 O (aq) → NR Fe(s) + Cu. SO 4(aq) → Fe. SO 4(aq) + Cu(s) Na(s) + Sn. CO 3(aq) → Na 2 CO 3(aq) + Sn(s)
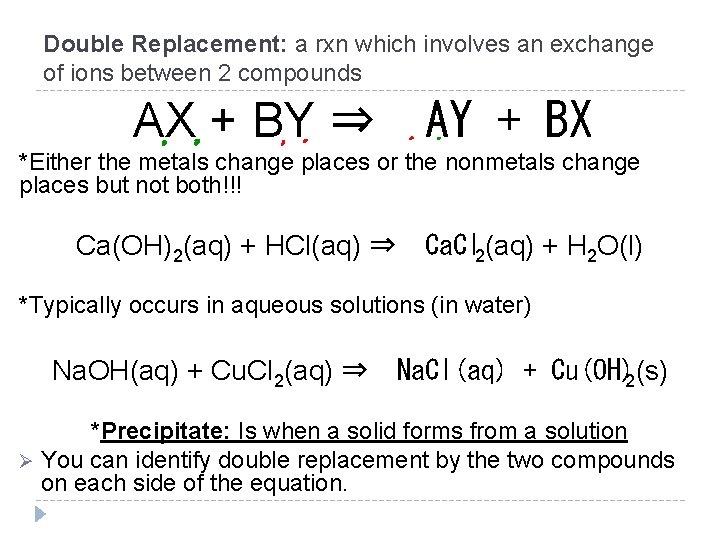
Double Replacement: a rxn which involves an exchange of ions between 2 compounds AX + BY ⇒ AY + BX *Either the metals change places or the nonmetals change places but not both!!! Ca(OH)2(aq) + HCl(aq) ⇒ Ca. Cl 2(aq) + H 2 O(l) *Typically occurs in aqueous solutions (in water) Na. OH(aq) + Cu. Cl 2(aq) ⇒ Na. Cl(aq) + Cu(OH)2(s) *Precipitate: Is when a solid forms from a solution Ø You can identify double replacement by the two compounds on each side of the equation.

3 Types of Double Replacement: 1. Formation of a precipitate 2. Formation of H 2 O 3. Formation of a product which decomposes into a gas
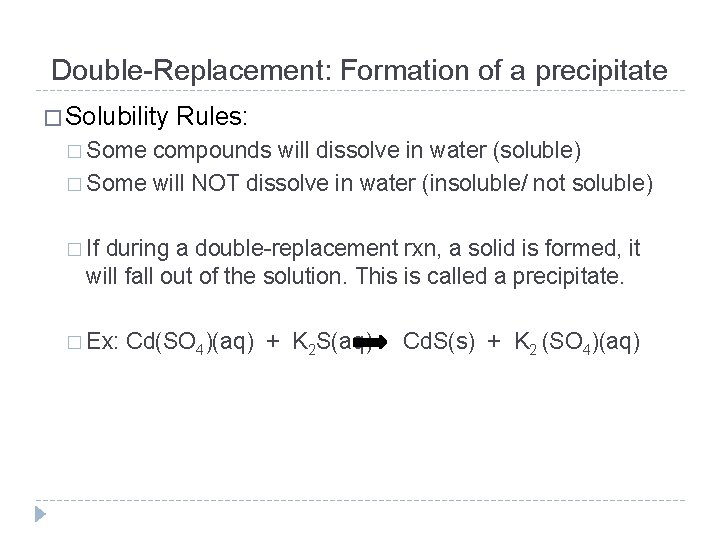
Double-Replacement: Formation of a precipitate � Solubility Rules: � Some compounds will dissolve in water (soluble) � Some will NOT dissolve in water (insoluble/ not soluble) � If during a double-replacement rxn, a solid is formed, it will fall out of the solution. This is called a precipitate. � Ex: Cd(SO 4)(aq) + K 2 S(aq) Cd. S(s) + K 2 (SO 4)(aq)
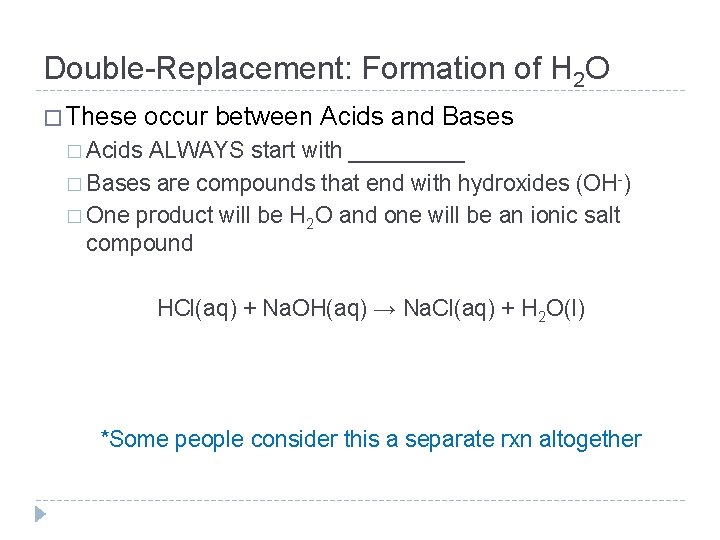
Double-Replacement: Formation of H 2 O � These occur between Acids and Bases � Acids ALWAYS start with _____ � Bases are compounds that end with hydroxides (OH-) � One product will be H 2 O and one will be an ionic salt compound HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l) *Some people consider this a separate rxn altogether
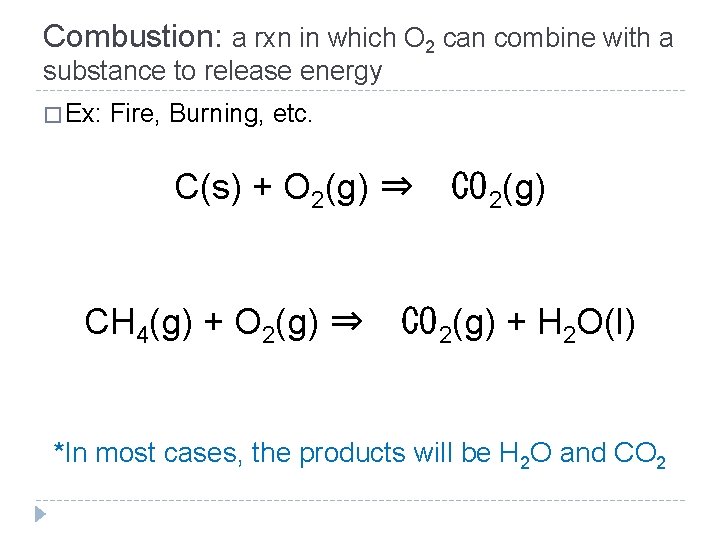
Combustion: a rxn in which O 2 can combine with a substance to release energy � Ex: Fire, Burning, etc. C(s) + O 2(g) ⇒ CH 4(g) + O 2(g) ⇒ CO 2(g) + H 2 O(l) *In most cases, the products will be H 2 O and CO 2
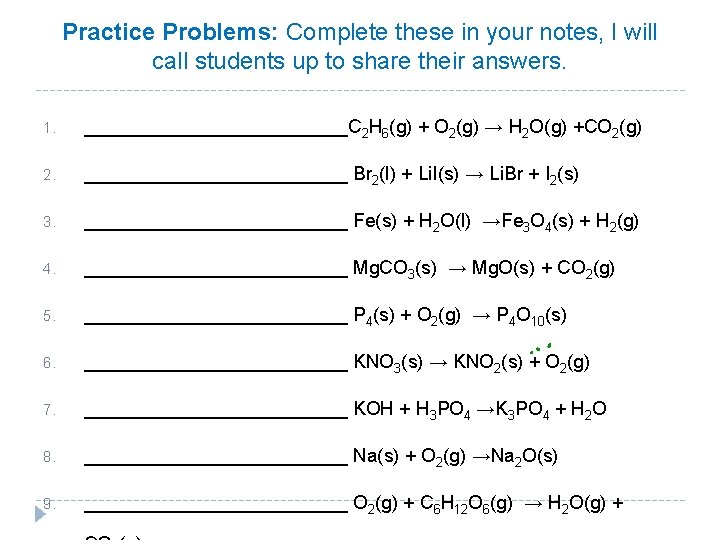
Practice Problems: Complete these in your notes, I will call students up to share their answers. 1. _____________C 2 H 6(g) + O 2(g) → H 2 O(g) +CO 2(g) 2. _____________ Br 2(l) + Li. I(s) → Li. Br + I 2(s) 3. _____________ Fe(s) + H 2 O(l) →Fe 3 O 4(s) + H 2(g) 4. _____________ Mg. CO 3(s) → Mg. O(s) + CO 2(g) 5. _____________ P 4(s) + O 2(g) → P 4 O 10(s) 6. _____________ KNO 3(s) → KNO 2(s) + O 2(g) 7. _____________ KOH + H 3 PO 4 →K 3 PO 4 + H 2 O 8. _____________ Na(s) + O 2(g) →Na 2 O(s) 9. _____________ O 2(g) + C 6 H 12 O 6(g) → H 2 O(g) +
- Slides: 12