Types of Chemical Reactions Chemical Equations and Reactions













- Slides: 17

Types of Chemical Reactions { Chemical Equations and Reactions In this part of chapter 8 we want to learn how to identify the type of chemical reaction that takes place, as well as how to predict what products will form as the result of the chemical reaction.

There are 5 main types of chemical reactions. 1. Synthesis 2. Decomposition 3. Single Displacement (Replacement) 4. Double Displacement (Replacement) 5. Combustion

Synthesis (Composition) Reactions Two or more substances combine to form a new compound. A + X AX Reaction of elements with oxygen and sulfur Reactions of metals with Halogens Synthesis reactions with Oxides There are others not covered here!

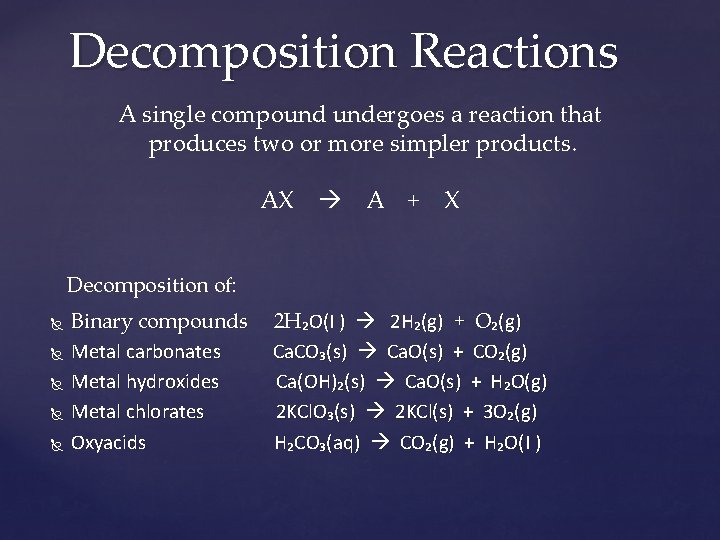
Decomposition Reactions A single compound undergoes a reaction that produces two or more simpler products. AX A + X Decomposition of: Binary compounds Metal carbonates Metal hydroxides Metal chlorates Oxyacids 2 H₂O(I ) 2 H₂(g) + O₂(g) Ca. CO₃(s) Ca. O(s) + CO₂(g) Ca(OH)₂(s) Ca. O(s) + H₂O(g) 2 KCl. O₃(s) 2 KCl(s) + 3 O₂(g) H₂CO₃(aq) CO₂(g) + H₂O(I )

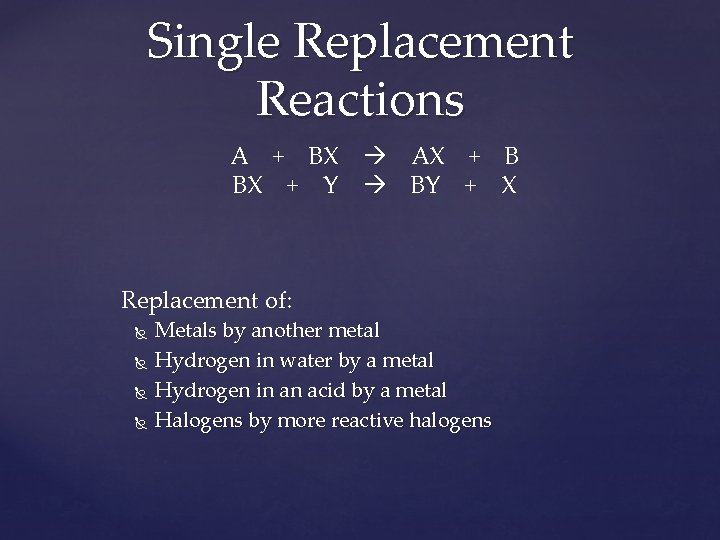
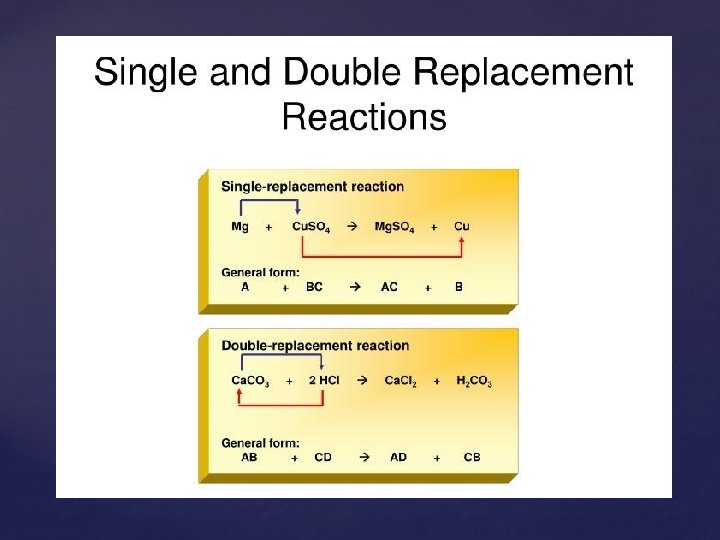
Single Replacement Reactions A + BX BX + Y Replacement of: AX + B BY + X Metals by another metal Hydrogen in water by a metal Hydrogen in an acid by a metal Halogens by more reactive halogens



By understanding how reactions work, we can predict what products will form.

Answers from previous slide Fe(s) + Cu. SO₄(aq) → Fe. SO₄(aq) + Cu(s) 2 KCl + Pb <-> Pb. Cl 2 + 2 K 2 Al(s) + 6 HCl(aq) = 2 Al. Cl 3(aq) + 3 H 2(g)

Double Replacement Reactions The ions of two compounds exchange places in an aqueous solution to form two new compounds AX + BY AY + BX One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually water.




Answers from the previous slide 2 Ag. NO 3 + K 2 Cr. O 4 -> Ag 2 Cr. O 4 + 2 KNO 3 2 KI + Pb(NO 3)2 -> 2 K(NO)3 + Pb. I 2 Na. CN + H 2 SO 4 -> HCN + Na 2 SO 4 Ca(OH)2 (s) + 2 HCl(aq) -> Ca. Cl 2(aq) + 2 H 2 O (l)

Combustion Reactions A substance combines with oxygen, releasing a large amount of energy in the form of light and heat. Reactive elements combine with oxygen P₄(s) + 5 O₂(g) P₄O₁₀(s) (This is also a synthesis reaction) The burning of natural gas, wood, gasoline, C₃H₈(g) +5 O₂(g) 3 CO₂(g) + 4 H₂O(g)

Self Assessment – Identifying Types of Chemical Reactions, Predicting Products &Writing Balanced Chemical Equations 1. nitrogen dioxide 2. glucose (C 6 H 12 O 6) + O 2 → 3. silver nitrate + copper 4. sodium phosphate + potassium hydroxide 5. hydrogen + oxygen

Answers 1. __2_ NO 2 → ___ N 2 + _2_ O 2 (decomposition) 2. ___ C 6 H 12 O 6 + __6_ O 2 → _6_ CO 2 + _6_ H 2 O (combustion) 3. _2_ Ag. NO 3 + ___Cu → ___ Cu(NO 3)2 + __2_ Ag (single replacement) 4. __ Na 3 PO 4 + _3_ KOH → ___K 3 PO 4 + _3_ Na. OH (double replacement) 5. __2__ H 2 + ____O 2 -> __2__H 2 O (synthesis)
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review I intro
I intro Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Section 1 chemical changes
Section 1 chemical changes Translating chemical equations
Translating chemical equations Types of chemical reactions redox
Types of chemical reactions redox Types of reaction
Types of reaction