Types of Chemical Reactions Chapter 8 Types of

- Slides: 6

Types of Chemical Reactions Chapter 8

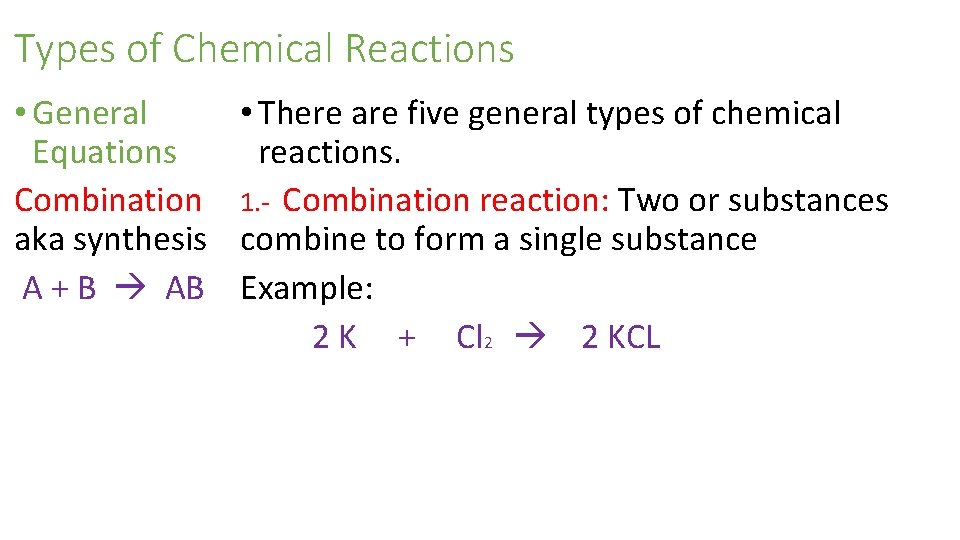
Types of Chemical Reactions • General • There are five general types of chemical Equations reactions. Combination 1. - Combination reaction: Two or substances aka synthesis combine to form a single substance A + B AB Example: 2 K + Cl 2 2 KCL

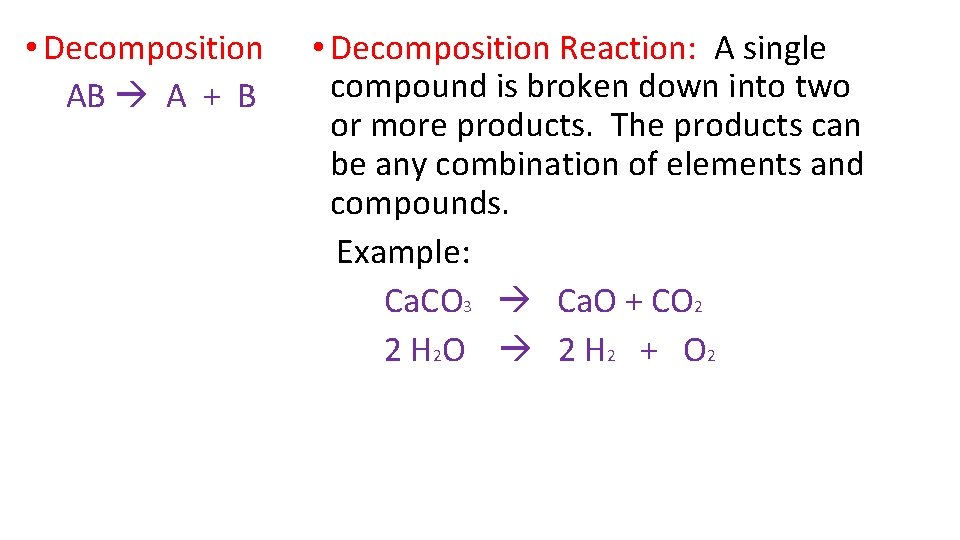
• Decomposition AB A + B • Decomposition Reaction: A single compound is broken down into two or more products. The products can be any combination of elements and compounds. Example: Ca. CO 3 Ca. O + CO 2 2 H 2 O 2 H 2 + O 2

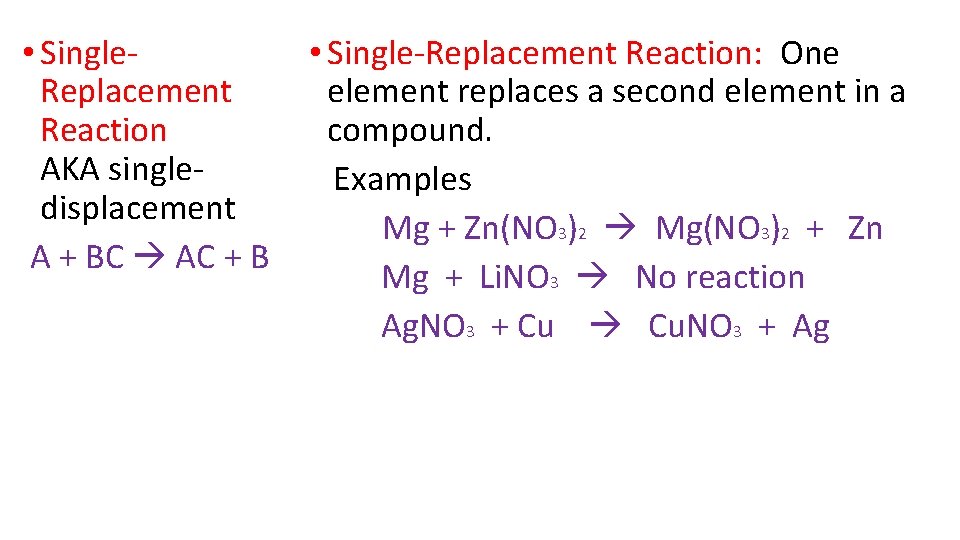
• Single. Replacement Reaction AKA singledisplacement A + BC AC + B • Single-Replacement Reaction: One element replaces a second element in a compound. Examples Mg + Zn(NO 3)2 Mg(NO 3)2 + Zn Mg + Li. NO 3 No reaction Ag. NO 3 + Cu Cu. NO 3 + Ag

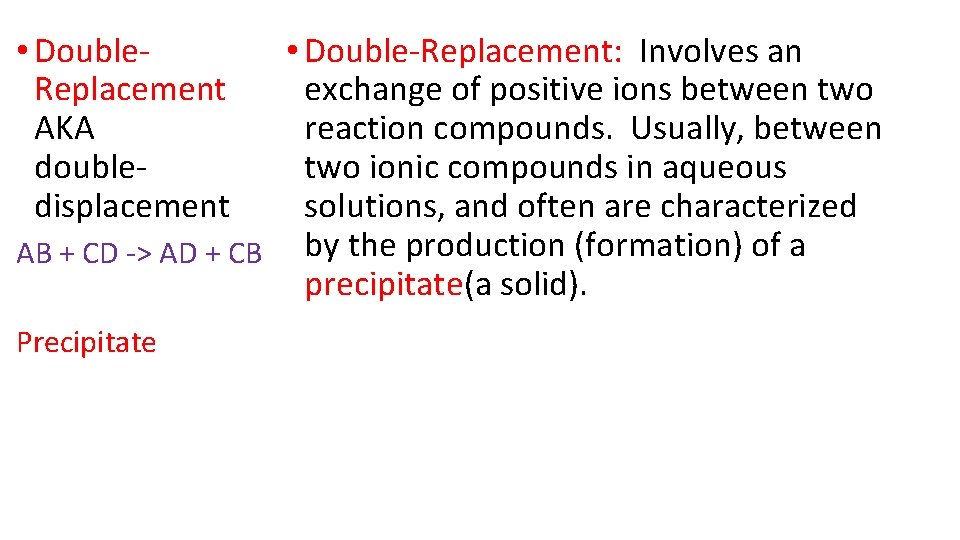
• Double. Replacement AKA doubledisplacement • Double-Replacement: Involves an exchange of positive ions between two reaction compounds. Usually, between two ionic compounds in aqueous solutions, and often are characterized AB + CD -> AD + CB by the production (formation) of a precipitate(a solid). Precipitate

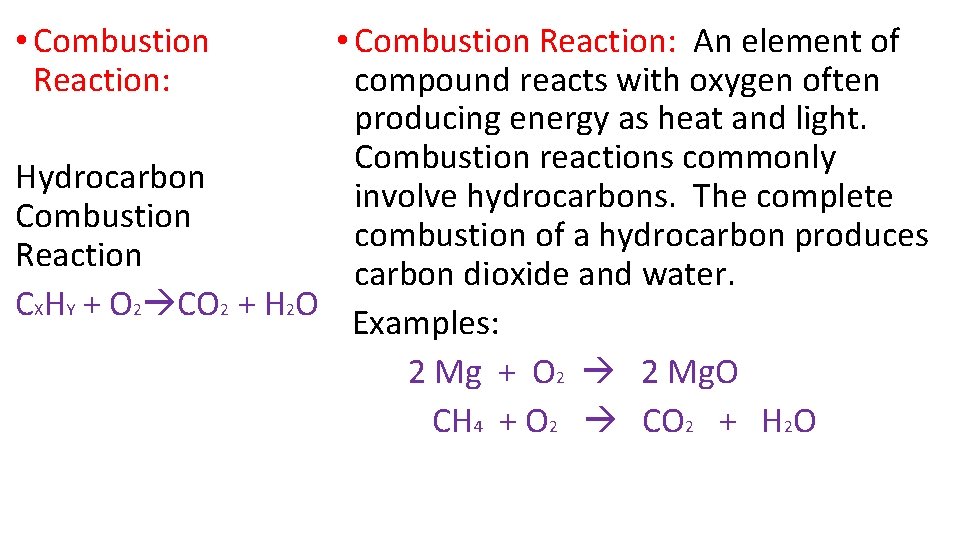
• Combustion Reaction: An element of compound reacts with oxygen often producing energy as heat and light. Combustion reactions commonly Hydrocarbon involve hydrocarbons. The complete Combustion combustion of a hydrocarbon produces Reaction carbon dioxide and water. CXHY + O 2 CO 2 + H 2 O Examples: 2 Mg + O 2 2 Mg. O CH 4 + O 2 CO 2 + H 2 O











