Types of Chemical Reactions 11 th Grade Honors





- Slides: 12

Types of Chemical Reactions 11 th Grade Honors Chemistry

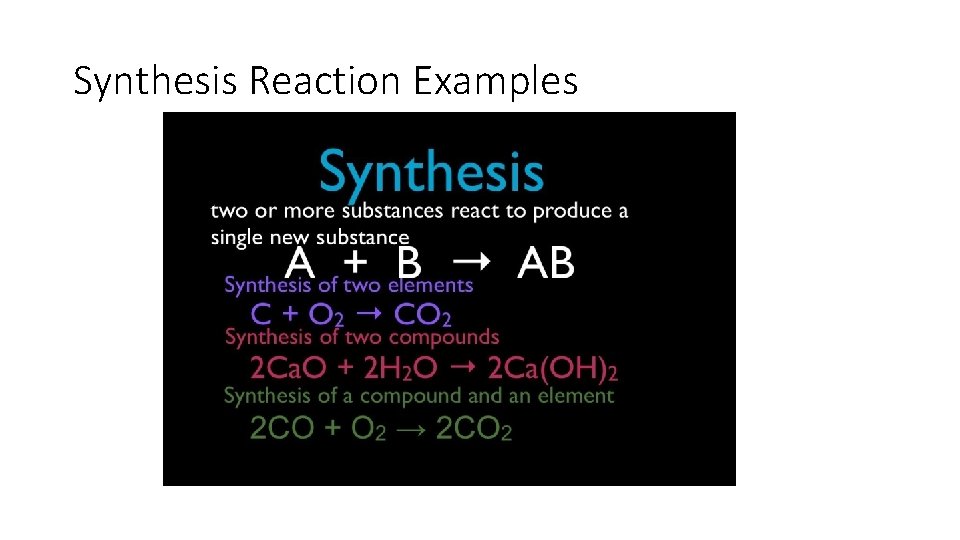
Synthesis Reactions • A synthesis reaction occurs when two or more reactants combine to form a single product. • A+B C

Synthesis Reaction Examples

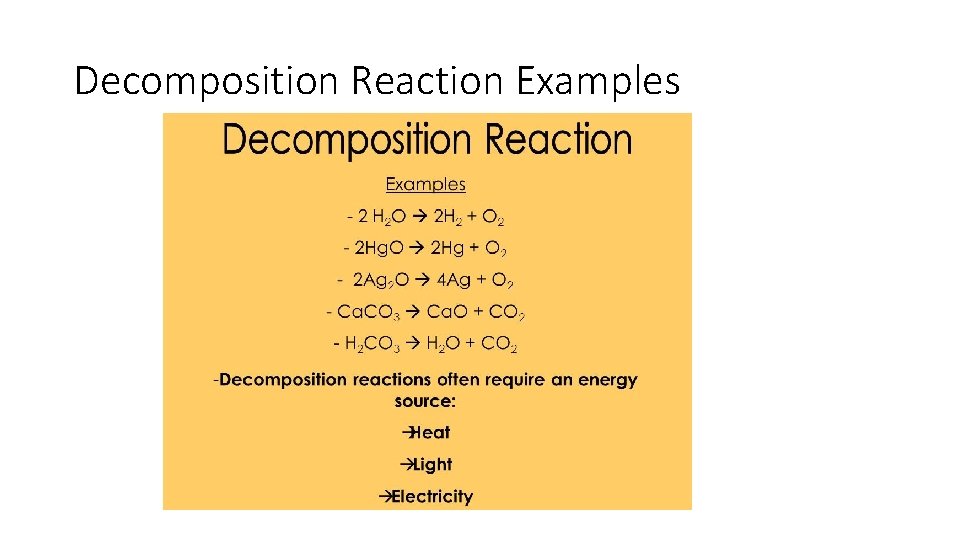
Decomposition Reactions • One reactant breaks down into two or more products. It is the reverse of a synthesis reaction. AB A+B

Decomposition Reaction Examples

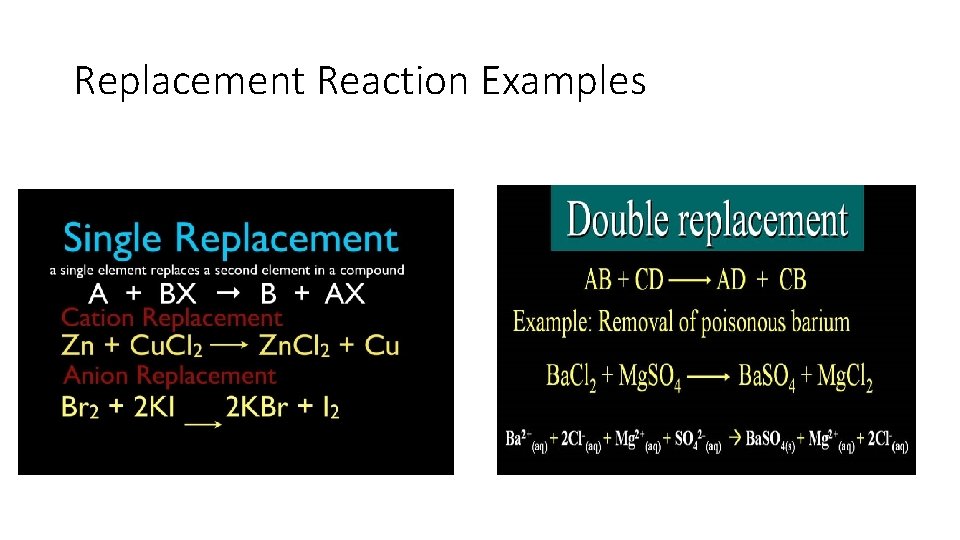
Replacement Reactions • Elements switch places in compounds. • In a single replacement reaction, one element takes the place of another in a single compound. • In a double replacement reaction, two compounds exchange elements.

Replacement Reaction Examples

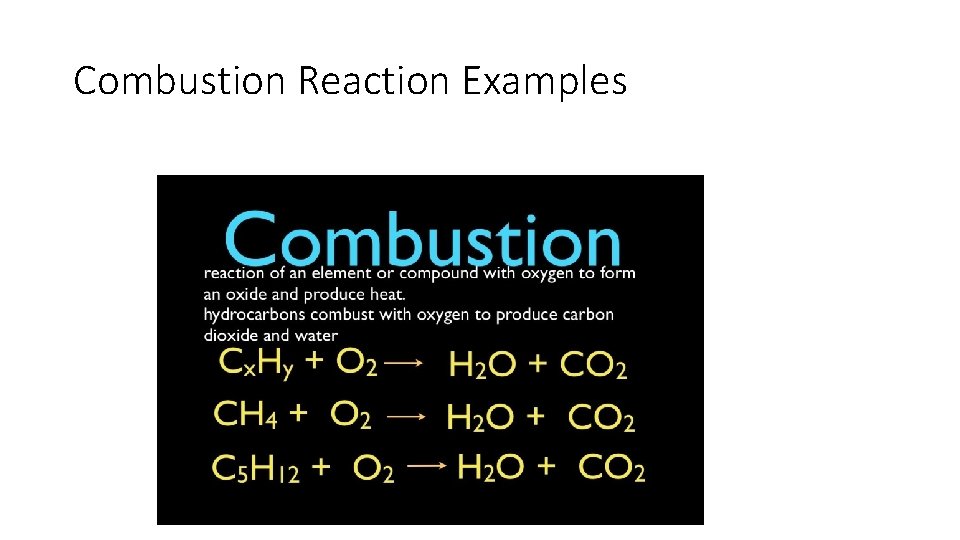
Combustion Reactions • When a substance reacts quickly with oxygen. • Combustion is commonly called “burning”. • Carbon Dioxide, water, and heat/light (it escapes) are all products of combustion.

Combustion Reaction Examples

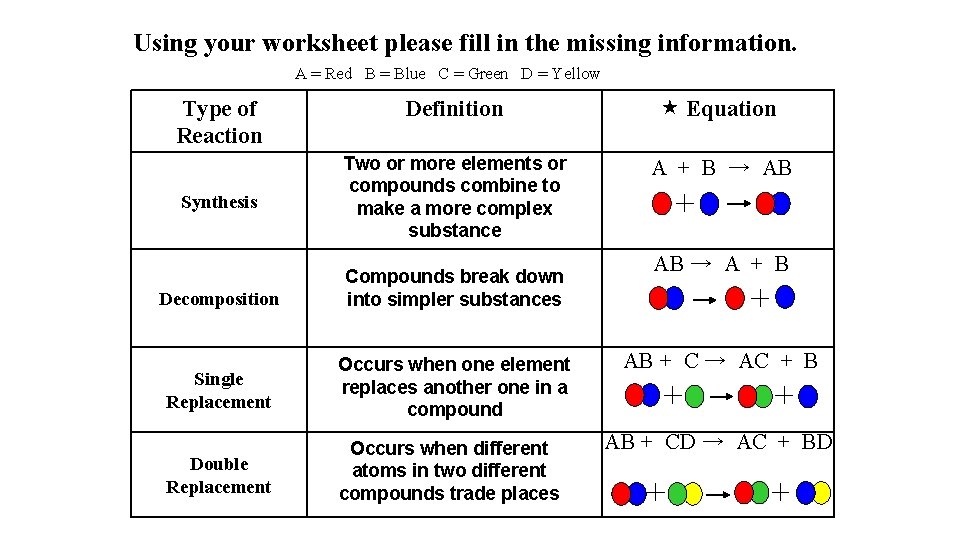
Using your worksheet please fill in the missing information. A = Red B = Blue C = Green D = Yellow Type of Reaction Synthesis Definition Equation Two or more elements or compounds combine to make a more complex substance A + B → AB AB → A + B Decomposition Compounds break down into simpler substances Single Replacement Occurs when one element replaces another one in a compound AB + C → AC + B Double Replacement Occurs when different atoms in two different compounds trade places AB + CD → AC + BD

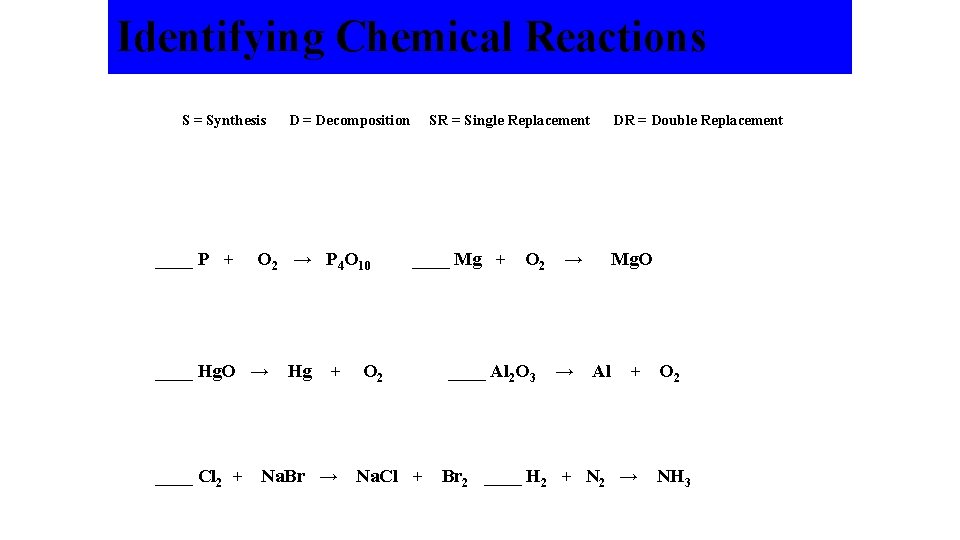
Identifying Chemical Reactions S = Synthesis ____ P + O 2 → P 4 O 10 ____ Hg. O → ____ Cl 2 + D = Decomposition Hg + Na. Br → O 2 SR = Single Replacement ____ Mg + DR = Double Replacement O 2 → ____ Al 2 O 3 → Mg. O Al + Na. Cl + Br 2 ____ H 2 + N 2 → O 2 NH 3

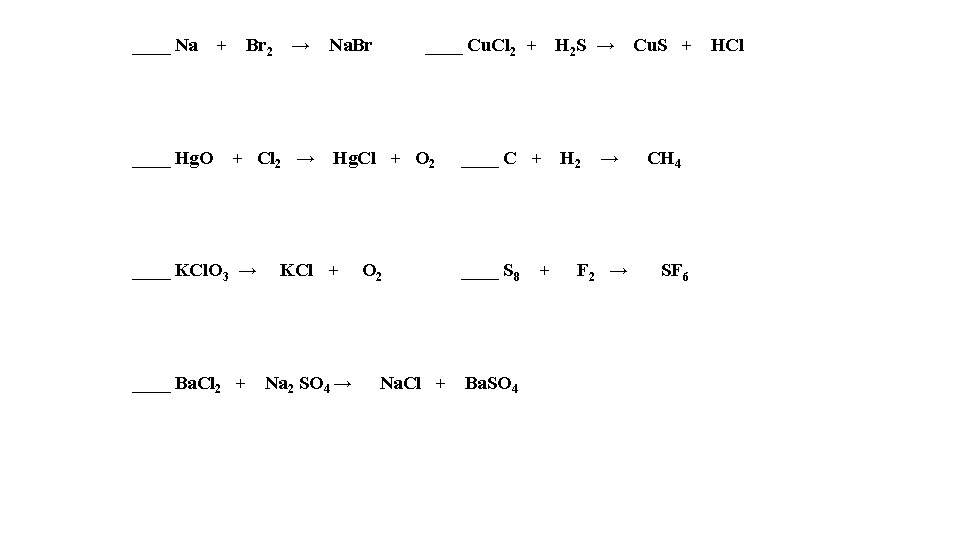
____ Na ____ Hg. O + Br 2 → + Cl 2 → Na. Br ____ Cu. Cl 2 + Hg. Cl + O 2 ____ KCl. O 3 → KCl + ____ Ba. Cl 2 + Na 2 SO 4 → O 2 ____ C + ____ S 8 + Na. Cl + Ba. SO 4 H 2 S → Cu. S + H 2 → CH 4 F 2 → SF 6 HCl