Types of Chemical Reaction Starter in pairs brainstorm

Types of Chemical Reaction Starter: in pairs brainstorm as many different TYPES of reaction as you know
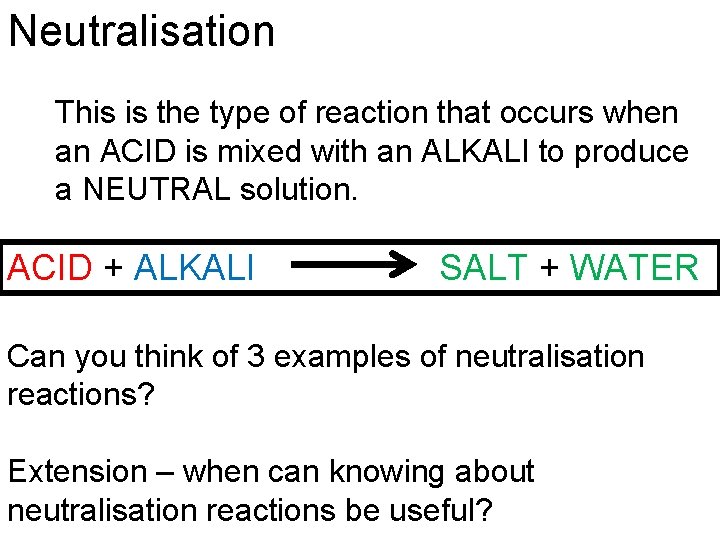
Neutralisation This is the type of reaction that occurs when an ACID is mixed with an ALKALI to produce a NEUTRAL solution. ACID + ALKALI SALT + WATER Can you think of 3 examples of neutralisation reactions? Extension – when can knowing about neutralisation reactions be useful?
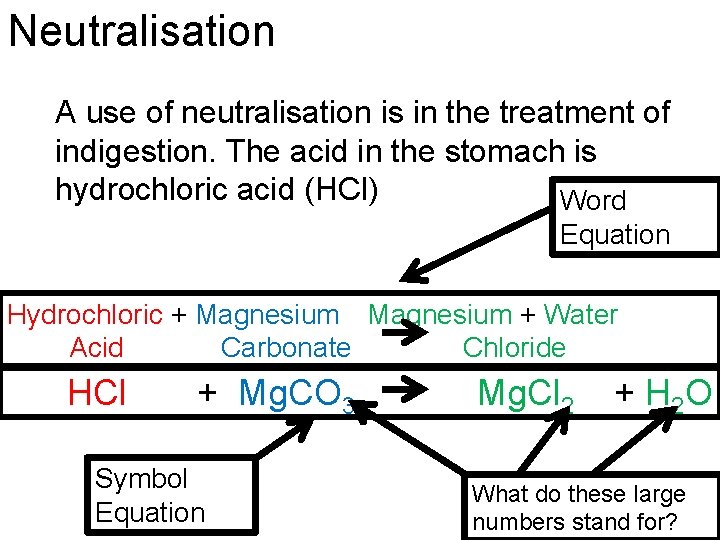
Neutralisation A use of neutralisation is in the treatment of indigestion. The acid in the stomach is hydrochloric acid (HCl) Word Equation Hydrochloric + Magnesium + Water Acid Carbonate Chloride HCl + Mg. CO 3 Symbol Equation Mg. Cl 2 + H 2 O What do these large numbers stand for?
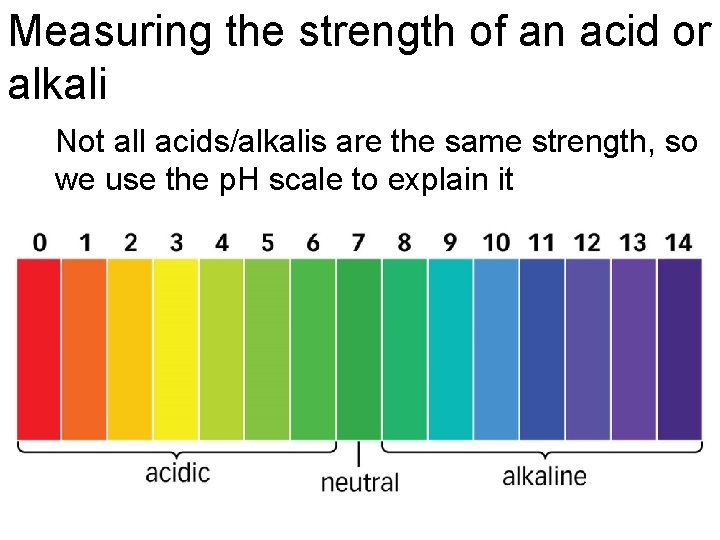
Measuring the strength of an acid or alkali Not all acids/alkalis are the same strength, so we use the p. H scale to explain it
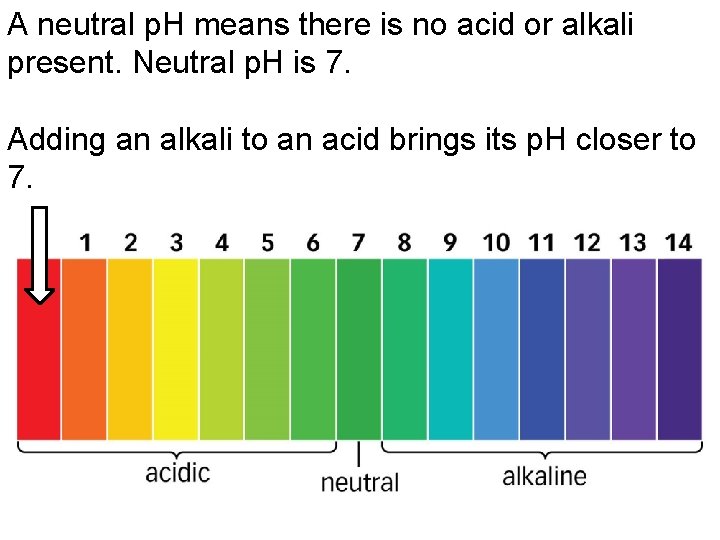
A neutral p. H means there is no acid or alkali present. Neutral p. H is 7. Adding an alkali to an acid brings its p. H closer to 7.
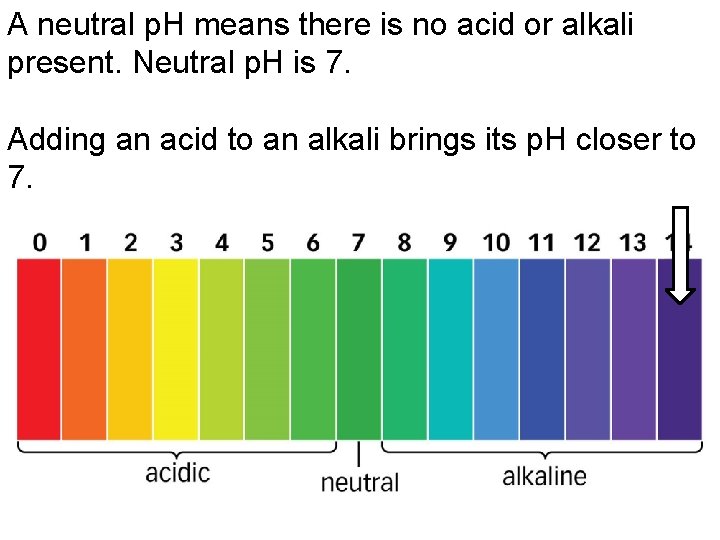
A neutral p. H means there is no acid or alkali present. Neutral p. H is 7. Adding an acid to an alkali brings its p. H closer to 7.

You have 3 antacids, their p. Hs are: • Antacid A – p. H 9 • Antacid B – p. H 13 • Antacid C – p. H 10. 5 Which of these will be better at neutralising the Hydrochloric Acid if you have to use the same amount of each? The extremes of the p. H scale are very harmful to human beings. Which antacids do you think you should rule out using because of this?

You have 3 antacids, the mass required to fully neutralise stomach acid is: • Antacid A – 84 g • Antacid B – 50 g • Antacid C – 70 g The cost per 100 g is: • Antacid A – 67 p • Antacid B – 98 p • Antacid C – 59 p Which of these is the most cost effective? What factors will affect the RRP of the antacid?
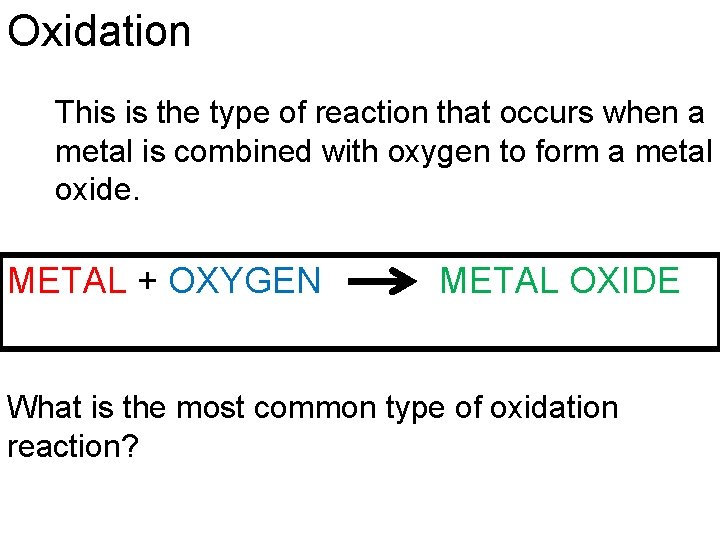
Oxidation This is the type of reaction that occurs when a metal is combined with oxygen to form a metal oxide. METAL + OXYGEN METAL OXIDE What is the most common type of oxidation reaction?
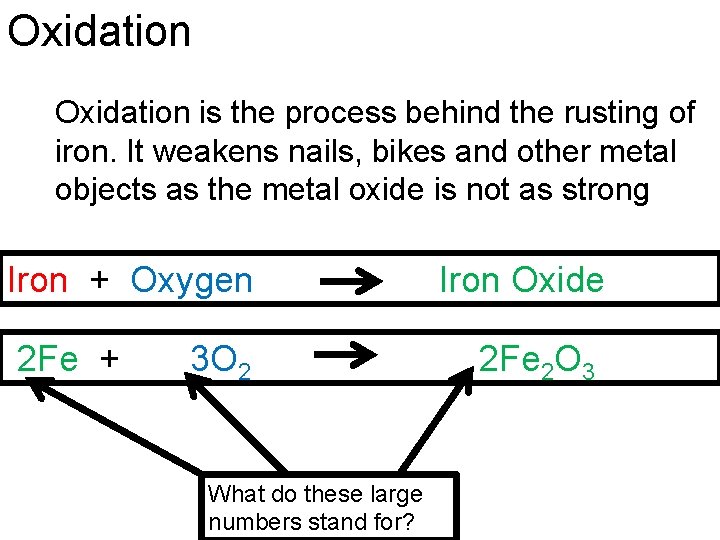
Oxidation is the process behind the rusting of iron. It weakens nails, bikes and other metal objects as the metal oxide is not as strong Iron + Oxygen 2 Fe + 3 O 2 What do these large numbers stand for? Iron Oxide 2 Fe 2 O 3
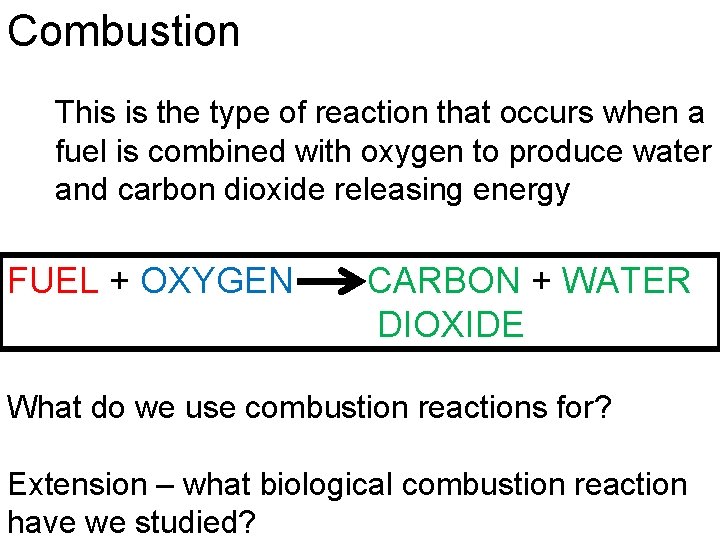
Combustion This is the type of reaction that occurs when a fuel is combined with oxygen to produce water and carbon dioxide releasing energy FUEL + OXYGEN CARBON + WATER DIOXIDE What do we use combustion reactions for? Extension – what biological combustion reaction have we studied?
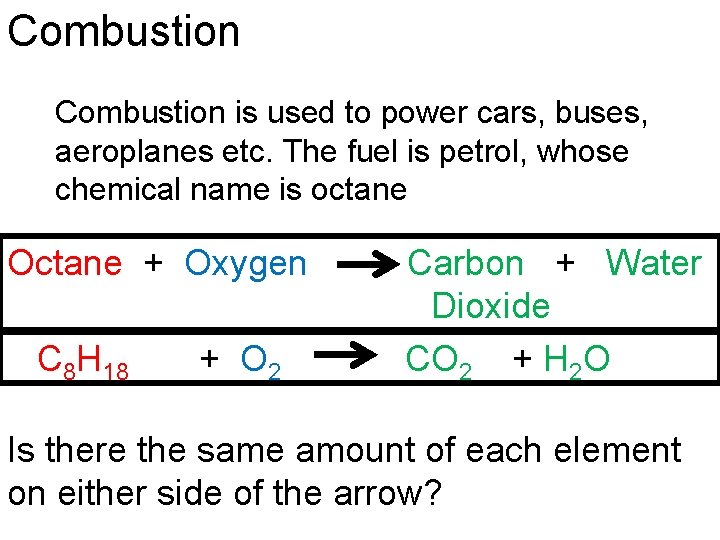
Combustion is used to power cars, buses, aeroplanes etc. The fuel is petrol, whose chemical name is octane Octane + Oxygen C 8 H 18 + O 2 Carbon + Water Dioxide CO 2 + H 2 O Is there the same amount of each element on either side of the arrow?
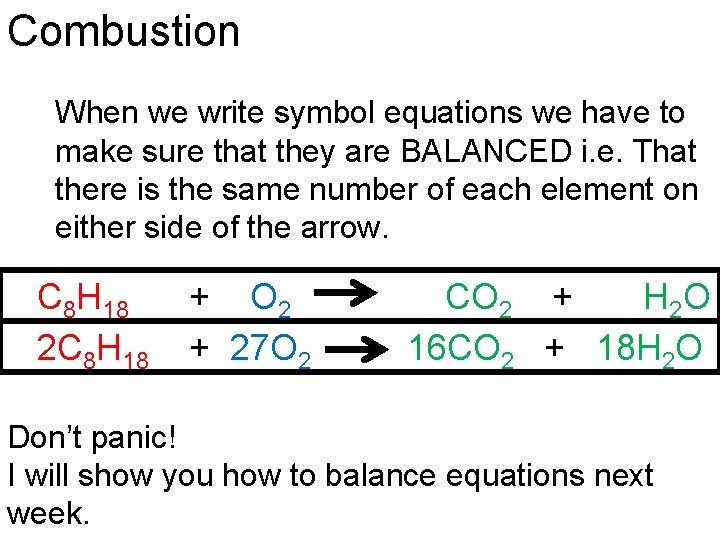
Combustion When we write symbol equations we have to make sure that they are BALANCED i. e. That there is the same number of each element on either side of the arrow. C 8 H 18 2 C 8 H 18 + O 2 + 27 O 2 CO 2 + H 2 O 16 CO 2 + 18 H 2 O Don’t panic! I will show you how to balance equations next week.
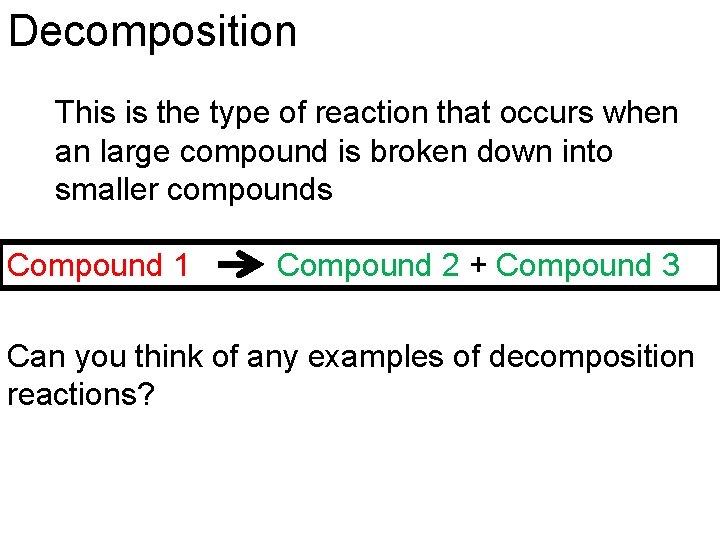
Decomposition This is the type of reaction that occurs when an large compound is broken down into smaller compounds Compound 1 Compound 2 + Compound 3 Can you think of any examples of decomposition reactions?
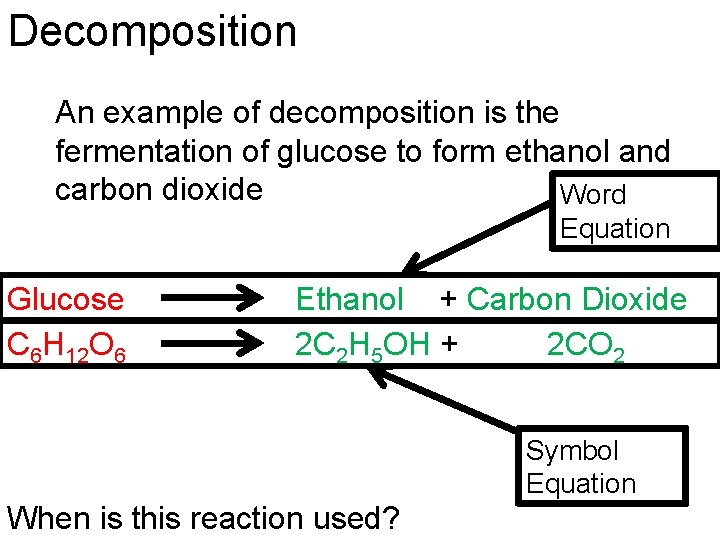
Decomposition An example of decomposition is the fermentation of glucose to form ethanol and carbon dioxide Word Equation Glucose C 6 H 12 O 6 Ethanol + Carbon Dioxide 2 C 2 H 5 OH + 2 CO 2 Symbol Equation When is this reaction used?
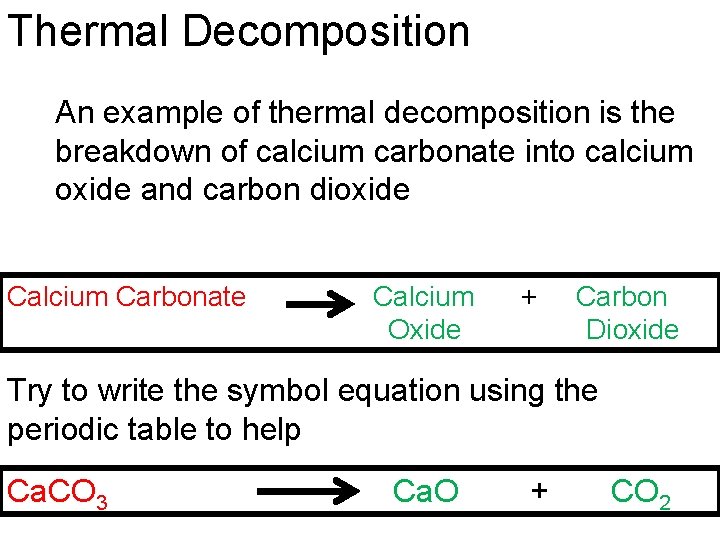
Thermal Decomposition An example of thermal decomposition is the breakdown of calcium carbonate into calcium oxide and carbon dioxide Calcium Carbonate Calcium Oxide + Carbon Dioxide Try to write the symbol equation using the periodic table to help Ca. CO 3 Ca. O + CO 2
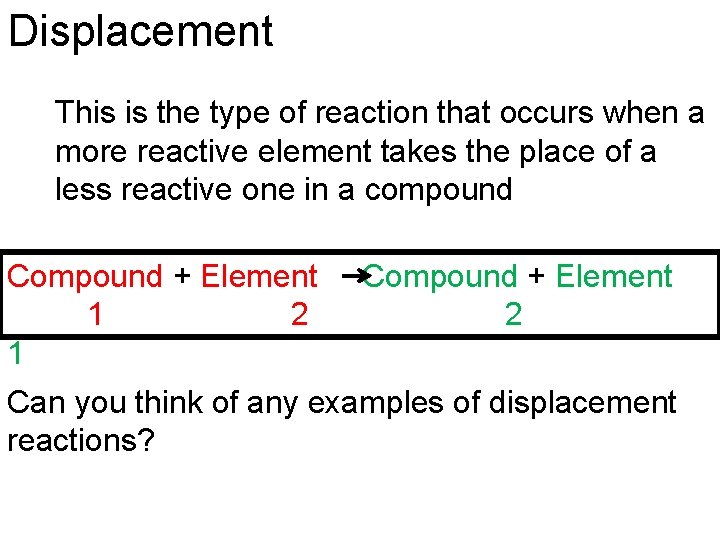
Displacement This is the type of reaction that occurs when a more reactive element takes the place of a less reactive one in a compound Compound + Element 1 2 1 Compound + Element 2 Can you think of any examples of displacement reactions?
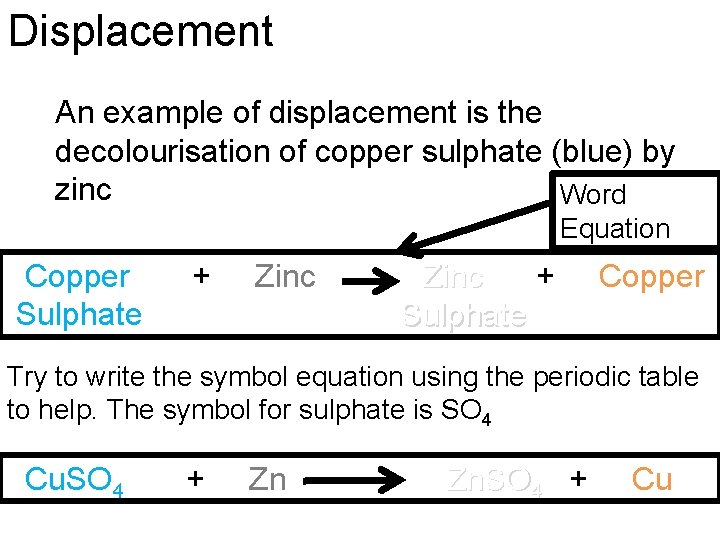
Displacement An example of displacement is the decolourisation of copper sulphate (blue) by zinc Word Equation Copper Sulphate + Zinc + Sulphate Copper Try to write the symbol equation using the periodic table to help. The symbol for sulphate is SO 4 Cu. SO 4 + Zn Zn. SO 4 + Cu
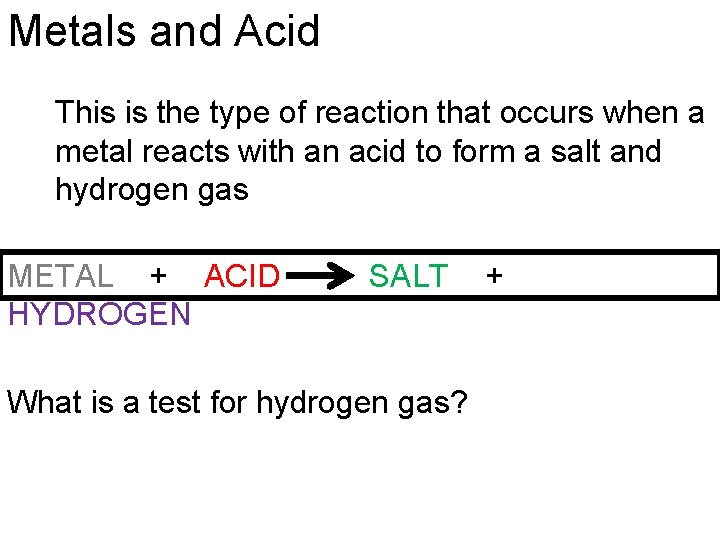
Metals and Acid This is the type of reaction that occurs when a metal reacts with an acid to form a salt and hydrogen gas METAL + ACID HYDROGEN SALT What is a test for hydrogen gas? +
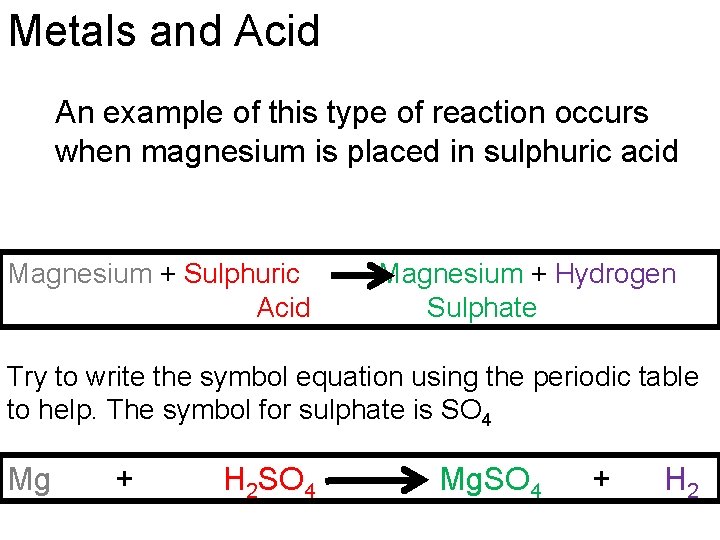
Metals and Acid An example of this type of reaction occurs when magnesium is placed in sulphuric acid Magnesium + Sulphuric Acid Magnesium + Hydrogen Sulphate Try to write the symbol equation using the periodic table to help. The symbol for sulphate is SO 4 Mg + H 2 SO 4 Mg. SO 4 + H 2
- Slides: 20