Types of chemical reaction Objectives To consider the
Types of chemical reaction

Objectives �To consider the range of different chemical reactions that there and their characteristics in terms of bonds, energy and products. �Outcomes: To know which types of chemical reactions we will be looking at in detail
The main types we have to learn � 1. Synthesis � 2. Decomposition reactions � 3. Simple substitution reactions � 4. Double substitution reactions � 5. Neutralisation reactions � 6. Combustion reactions
Synthesis (AKA Combination) � 2 or more elements and or compounds combine to form a single product. �A + B = C �E. g. S + O 2 = SO 2 �What is the word equation for this?
Decomposition reactions �A single reactant splits into two or more products: �A = B + C �E. g � 2 Hg. O = 2 Hg + O 2 �What is the word equation for this ?
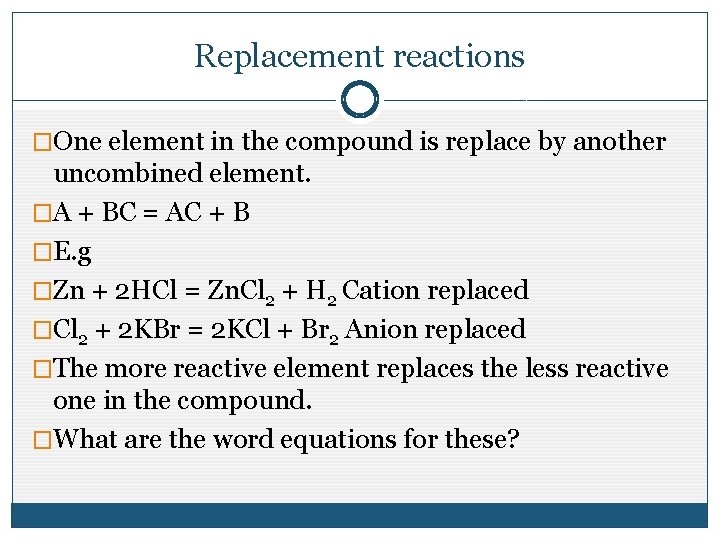
Replacement reactions �One element in the compound is replace by another uncombined element. �A + BC = AC + B �E. g �Zn + 2 HCl = Zn. Cl 2 + H 2 Cation replaced �Cl 2 + 2 KBr = 2 KCl + Br 2 Anion replaced �The more reactive element replaces the less reactive one in the compound. �What are the word equations for these?
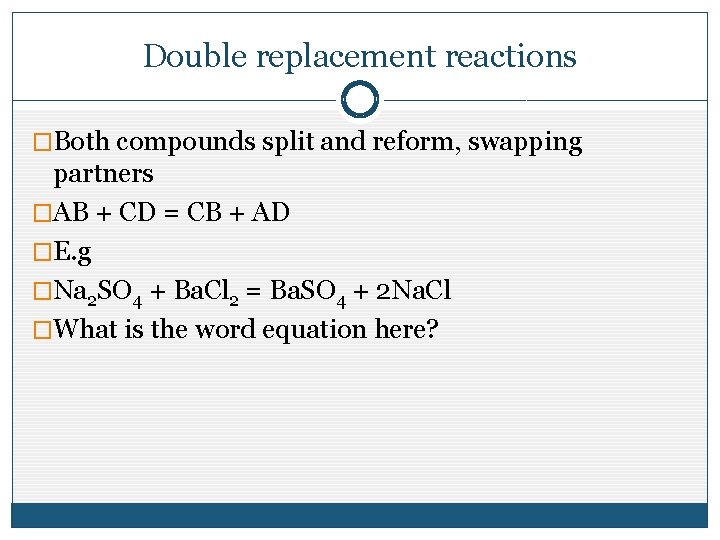
Double replacement reactions �Both compounds split and reform, swapping partners �AB + CD = CB + AD �E. g �Na 2 SO 4 + Ba. Cl 2 = Ba. SO 4 + 2 Na. Cl �What is the word equation here?
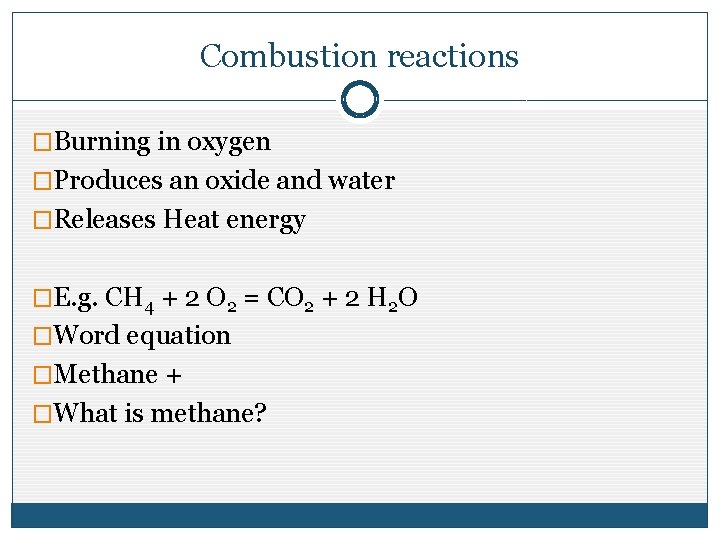
Combustion reactions �Burning in oxygen �Produces an oxide and water �Releases Heat energy �E. g. CH 4 + 2 O 2 = CO 2 + 2 H 2 O �Word equation �Methane + �What is methane?

Neutralisation �Acid + Alkali = Salt + Water �E. g. 2 Na. OH + H 2 SO 4 = Na 2 SO 4 + 2 H 2 O �What is this word eqaution? �Sulphuric acid makes sulphates �Nitric acid makes nitrates �Hydrochloric acids make chlorides �Carbonic acids make carbonate. �(Learn these!)
Find useful examples of each type of reaction � 1. Synthesis � 2. Decomposition reactions � 3. Simple substitution reactions � 4. Double substitution reactions � 5. Neutralisation reactions � 6. Combustion reactions �In your teams produce a report covering all of these– follow my rubric below
Rubric for types of reactions �Say what it is used for (3 marks) �Give a specific reaction with equation (3 marks) �Describe conditions needed for it to occur (3 marks) �Describe things like usefulness, amount of product produced, efficiency etc. (5 marks) �Explain any environmental impacts (2 marks) �Include visual info with captions (2 marks) �Write in good clear English – check each other’s contributions. (2 marks) �Once marked we will upload it to the Facebook page and add photos and further descriptions from the lab.
- Slides: 11