Types of Bonding Lab Wrap Up What Do

Types of Bonding Lab Wrap Up
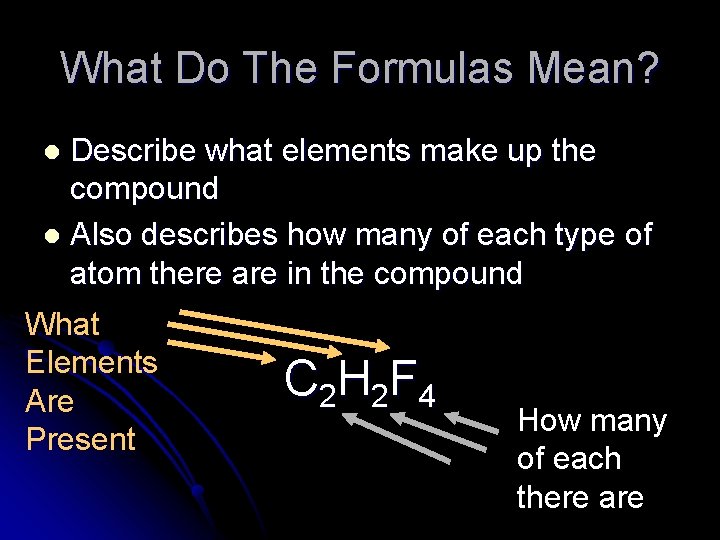
What Do The Formulas Mean? Describe what elements make up the compound l Also describes how many of each type of atom there are in the compound l What Elements Are Present C 2 H 2 F 4 How many of each there are

More Complicated Formulae l Formulae may have the same element more than once. This indicates something about the chemical structure, which we will learn about in Unit 5. l For example: NH 4 OH

More Complicated Formulae l Formulae may have parentheses: For example: Al(NO 3)3 The number outside the parentheses applies l to each of the atoms in the parentheses

How Many of Each Atom Are In A Formula? C 3 H 8 O Mg. SO 4 Ca(OH)2 NH 4 NO 3 Mg 3(PO 4)2 Fe(NO 3)3

What Does Conductivity Mean? How well a solution conducts electricity l What do you need to conduct electricity? l l To conduct electricity you need moving charges l Things with high conductivities have lots of things with charges that can move.

A Few Conductivity Terms l Electrolyte – a compound that conducts electricity when dissolved in water. Strong electrolyte – conducts electricity well l Weak electrolyte – conducts electricity slightly l Non-electrolyte – does not conduct electricity l

What does the Melting Point mean? l When a compound changes states some of the forces that attract the various pieces together are broken. l Low melting points indicate weak attractions between the little bits.

What Does Appearance Mean? l Regular Shapes are very important in science l A Regular Shape at the visible level means that there is a very Regular Shape at the microscopic and atomic levels.



Types of Chemical Bonding What Makes Salt, Aspirin, and Zinc Have Such Different Properties?

Things Like Sodium Chloride l l l High Melting Points Do Not Conduct Electricity in Solid Form Conduct Electricity in Solution Have a regular shape Made from a metal and a nonmetal (ex. Na. Cl)
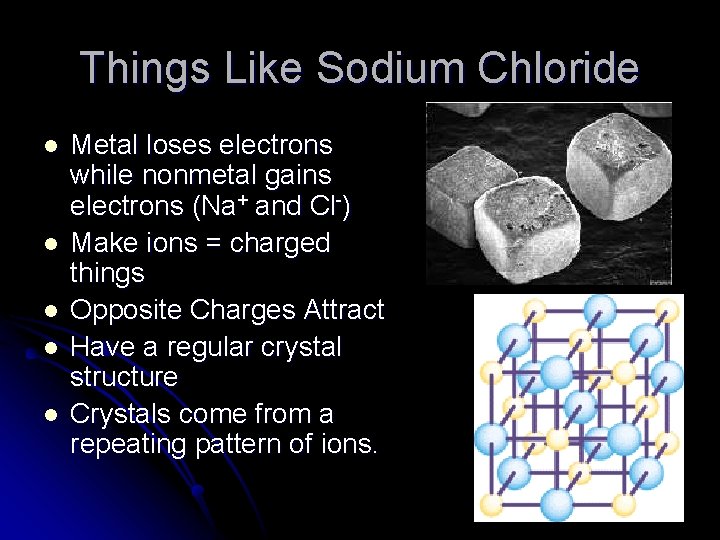
Things Like Sodium Chloride l l l Metal loses electrons while nonmetal gains electrons (Na+ and Cl-) Make ions = charged things Opposite Charges Attract Have a regular crystal structure Crystals come from a repeating pattern of ions.
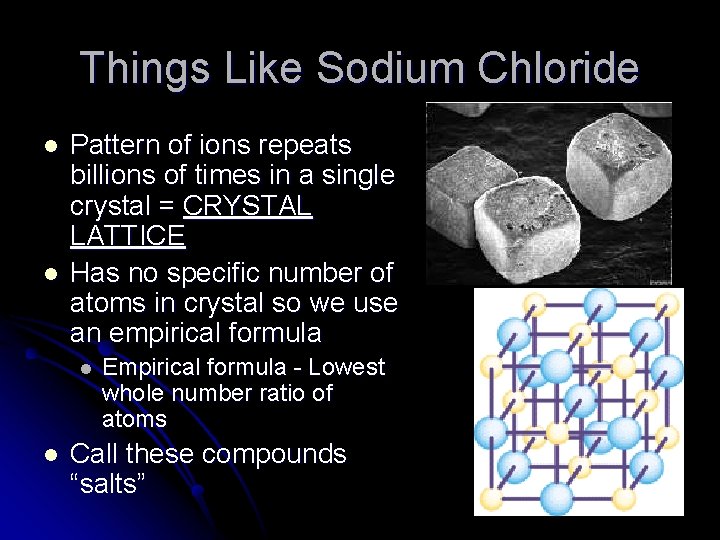
Things Like Sodium Chloride l l Pattern of ions repeats billions of times in a single crystal = CRYSTAL LATTICE Has no specific number of atoms in crystal so we use an empirical formula l l Empirical formula - Lowest whole number ratio of atoms Call these compounds “salts”
Things Like Sodium Chloride l l When melted, ions are freed from the crystal lattice structure Melting requires breaking the bonds holding the compound together l l Bonds are strong Therefore, melting point is high.
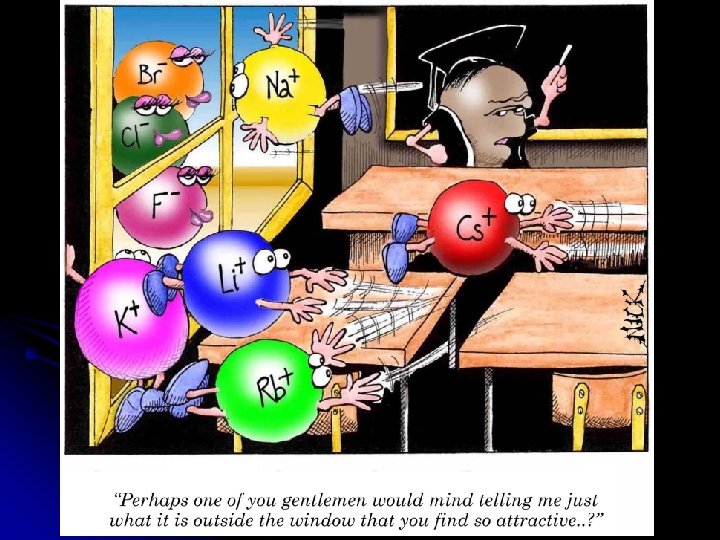

IONIC BONDING Bonding between ions Atoms give and take electrons Transfer electrons Things like Salt

Things Like Aspirin l l l Low Melting Points Do Not Conduct Electricity in Solid Form Most Do Not conduct electricity in aqueous solution (some compounds in this category do a little bit) Have an irregular shape Made from all nonmetals (ex. C 9 H 8 O 4)
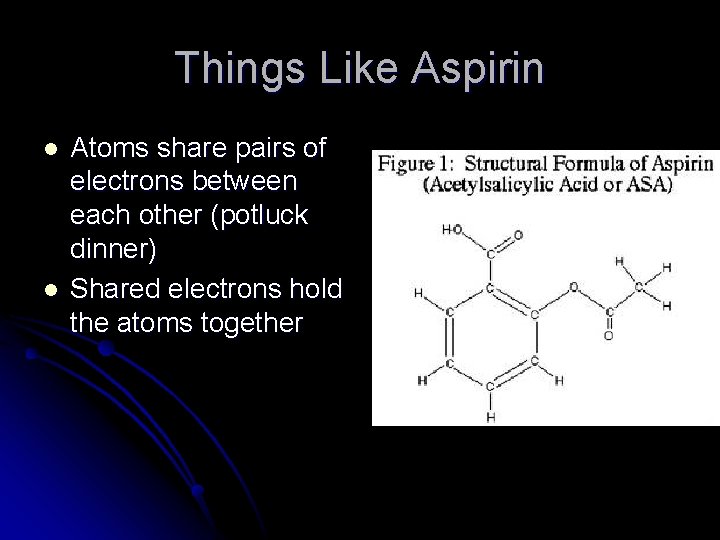
Things Like Aspirin l l Atoms share pairs of electrons between each other (potluck dinner) Shared electrons hold the atoms together
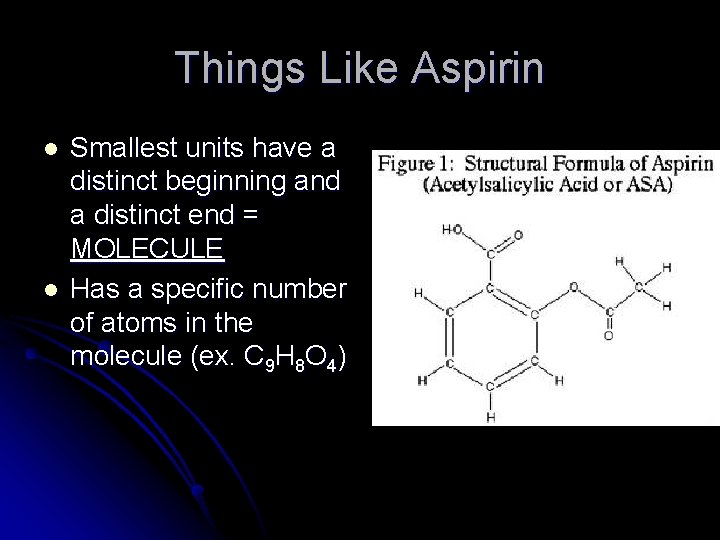
Things Like Aspirin l l Smallest units have a distinct beginning and a distinct end = MOLECULE Has a specific number of atoms in the molecule (ex. C 9 H 8 O 4)
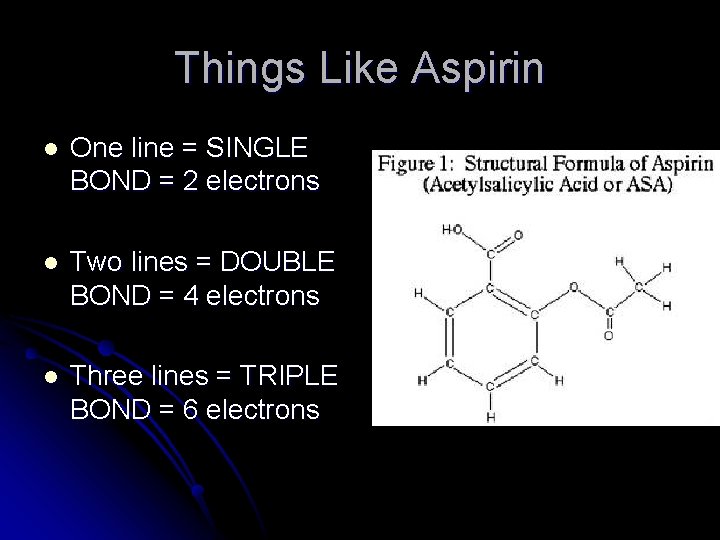
Things Like Aspirin l One line = SINGLE BOND = 2 electrons l Two lines = DOUBLE BOND = 4 electrons l Three lines = TRIPLE BOND = 6 electrons
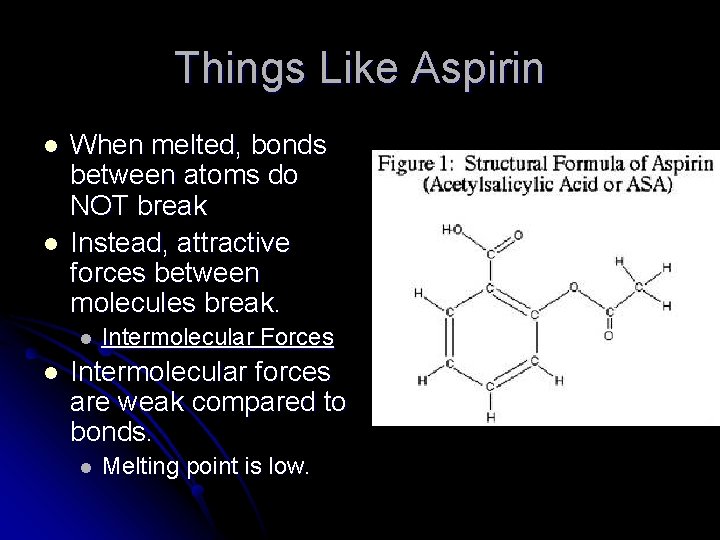
Things Like Aspirin l l When melted, bonds between atoms do NOT break Instead, attractive forces between molecules break. l l Intermolecular Forces Intermolecular forces are weak compared to bonds. l Melting point is low.

COVALENT BONDING Atoms Share Electrons to Make a Compound Things like Aspirin

Things Like Zinc l l l High Melting Points DO conduct electricity in the solid state Don’t form aqueous solutions Have irregular shape but are malleable Made from all metals (ex. Brass – a mixture of copper and zinc)
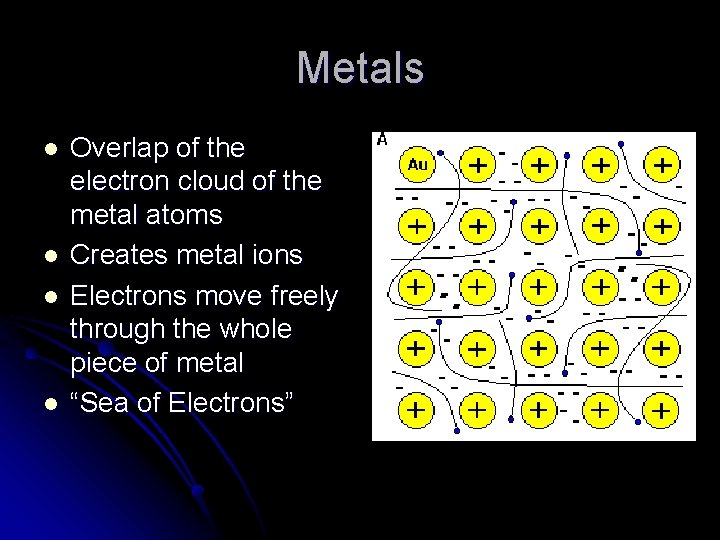
Metals l l Overlap of the electron cloud of the metal atoms Creates metal ions Electrons move freely through the whole piece of metal “Sea of Electrons”
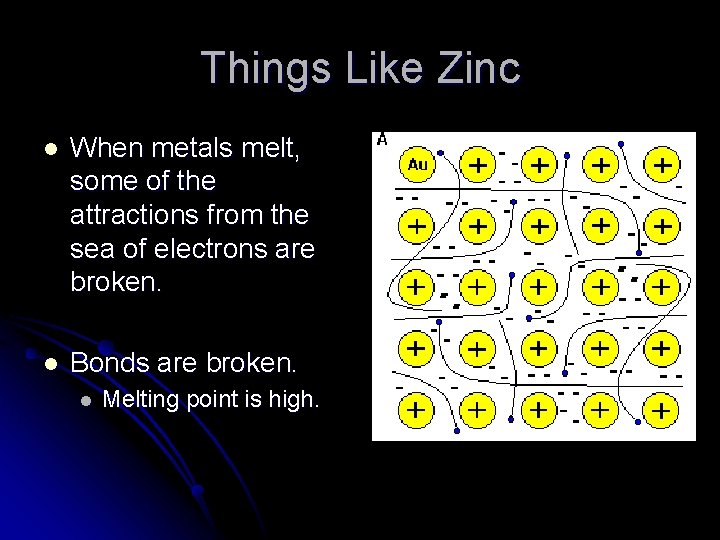
Things Like Zinc l When metals melt, some of the attractions from the sea of electrons are broken. l Bonds are broken. l Melting point is high.

Things Like Zinc Because of the sea of electrons metals: l Can have its shape changed = MALLEABLE l Can be pulled into wire = DUCTILE l Are shiny = LUSTEROUS

METALLIC BONDING A Sea of Electron Glue Holds Atoms Together Delocalized Electrons Things like Zinc
- Slides: 29