Types of Bonding in Solids Purpose Observe physical

Types of Bonding in Solids

Purpose • Observe physical properties of solids • Determine the type of bond in the solids using knowledge of the properties

Pre-lab • Using the second page of the lab follow the instructions to make a table that summarizes the properties of ionic, molecular, metallic and covalent network solids. • Include columns for: • • Name Definition Two examples of solids with this bond Solubility in water Ability to conduct as a solid and dissolved in water Relative Melting points (high, low, etc) Appearance

Procedure • Follow the procedure only completing one compound at a time. • See lab handout and images for set-up to follow
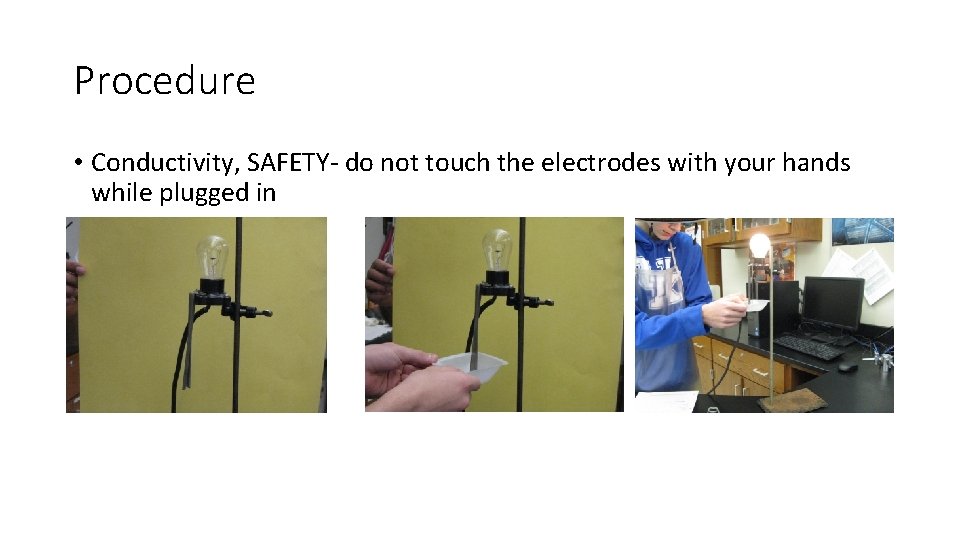
Procedure • Conductivity, SAFETY- do not touch the electrodes with your hands while plugged in

Relative Melting Point • Do NOT heat for more than 5 minutes. At the 1 st sign of melting stop heating and record the time.

Solubility and conductivity in solution • Measure. 5 grams of solid and add 5 m. L of distilled water. Shake and then check if the solid dissolved. We use distilled water because it doesn’t conduct electricity on its own- tap water does conduct electricity

Analysis and Conclusions • Compare the behavior of each solid with the pre-lab research chart to classify each solid and the unknown as • • Ionic Molecular Metallic Covalent Network
- Slides: 8