Types of Bonding and Lewis Structures Describe the
Types of Bonding and Lewis Structures

Describe the structure of metallic bonding. • Positive metallic ions surrounded by electrons.
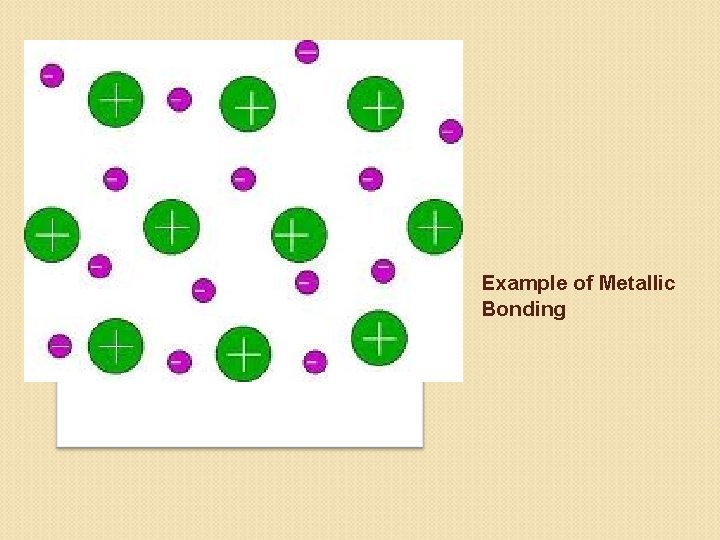
Example of Metallic Bonding

Potassium bromide forms a crystal lattice structure. What type of bonding would you expect it have? Ionic

Which of these elements would make a covalent bond with nitrogen? Al Mg • C; Ba C non-metal with non-metal bonding (also similar electronegativity)

Which of these elements would make an ionic bond with potassium? Mg • N, N Sr Cl Cl; metal with non-metal bond (also difference in electronegativities is relatively large)

Which of these elements would make an ionic bond with chlorine? O • Li; F Li Xe metal with non-metal bond (also difference in electronegativities is relatively large)

What type of bond exists between chlorine (EN = 3. 0) and bromine (EN = 2. 8) • Non-polar covalent; both elements are non-metals with their difference in electronegativity being less than 0. 5

What type of bond exists between nitrogen (EN = 3. 0) and hydrogen (EN = 2. 1) • Polar covalent; both elements are non-metals with their difference in electronegativity being between 0. 5 and 1. 7
Draw the correct Lewis dot structure for CH 2 O
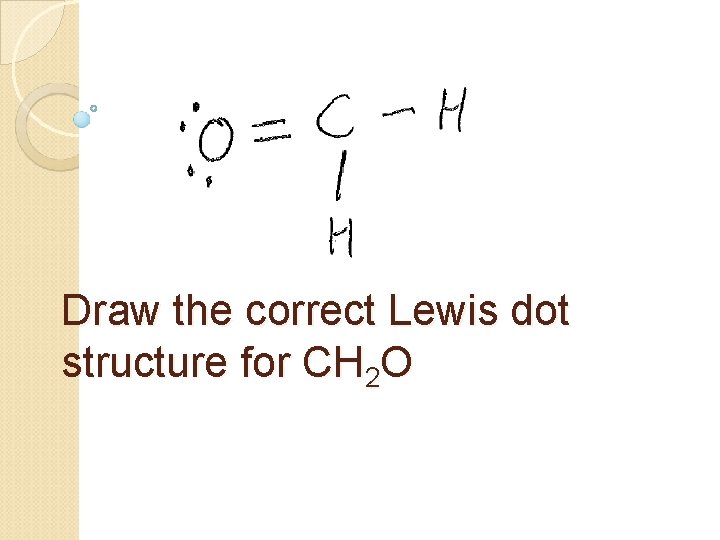
Draw the correct Lewis dot structure for CH 2 O

Draw the correct Lewis dot structure for SCl 2
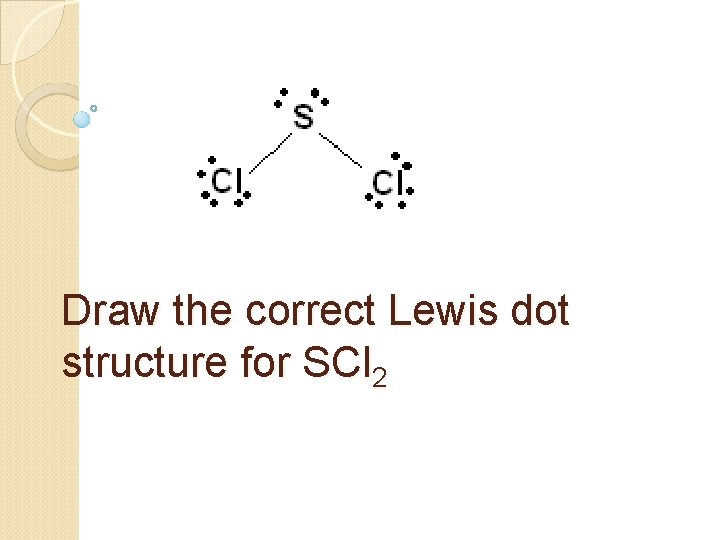
Draw the correct Lewis dot structure for SCl 2
Draw the correct Lewis Dot Structure for O 2
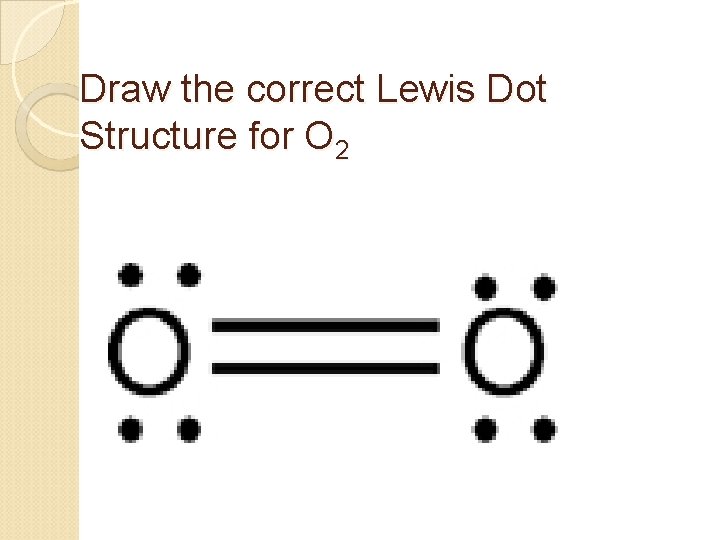
Draw the correct Lewis Dot Structure for O 2

Electron Configuration Review Questions

Write the electron configuration for the element fluorine • 1 s 22 p 5

Write the electron configuration for the element potassium • 1 s 22 p 63 s 23 p 64 s 1

Write the electron configuration for the element silicon • 1 s 22 p 63 s 23 p 2

Write the electron configuration for the element titanium • 1 s 22 p 63 s 23 p 64 s 23 d 2
![Write the noble gas configuration for the element rubidium • [Kr]5 s 1 Write the noble gas configuration for the element rubidium • [Kr]5 s 1](http://slidetodoc.com/presentation_image/2cdf9f5ab717fbddc8739765aa5d8141/image-21.jpg)
Write the noble gas configuration for the element rubidium • [Kr]5 s 1
![Write the noble gas configuration for the element chlorine • [Ne]3 s 2 3 Write the noble gas configuration for the element chlorine • [Ne]3 s 2 3](http://slidetodoc.com/presentation_image/2cdf9f5ab717fbddc8739765aa5d8141/image-22.jpg)
Write the noble gas configuration for the element chlorine • [Ne]3 s 2 3 p 5

Which element has the following electron configuration: 1 s 22 p 63 s 23 p 64 s 23 d 6 • Iron, Fe

Which element has the following electron configuration: 1 s 22 p 63 s 23 p 64 s 23 d 104 p 3 • Arsenic, As
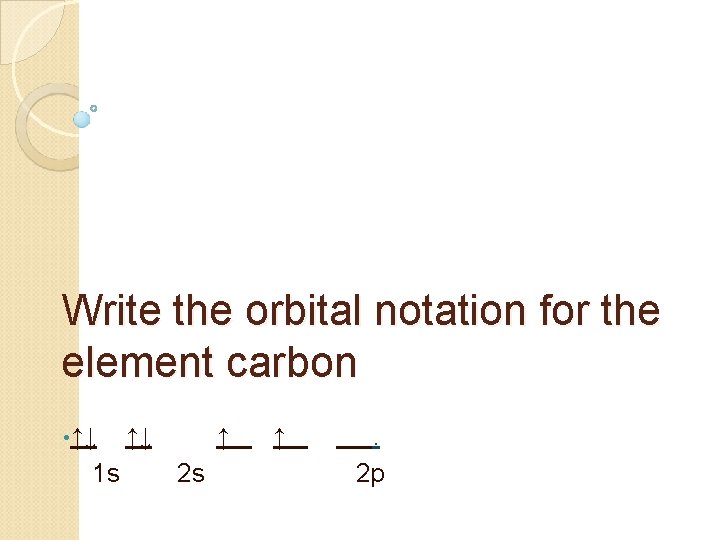
Write the orbital notation for the element carbon • ↑↓ 1 s ↑↓ ↑ 2 s ↑ . 2 p
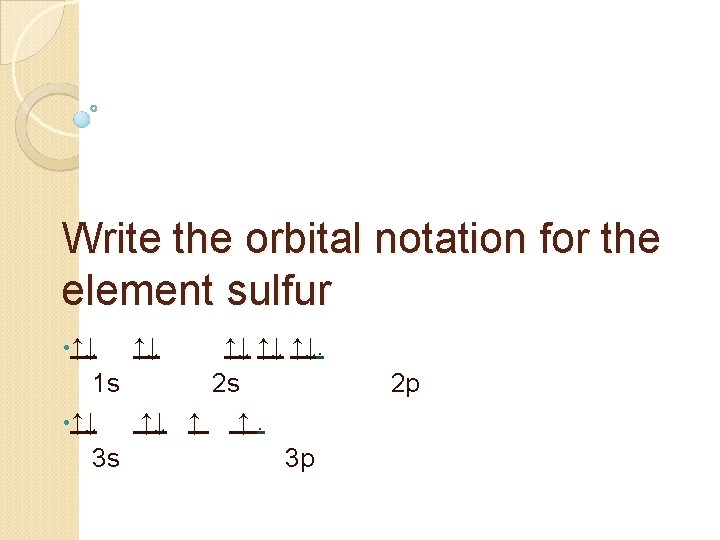
Write the orbital notation for the element sulfur • ↑↓ ↑↓ ↑↓. 1 s 2 s • ↑↓ ↑↓ ↑ ↑. 3 s 3 p 2 p

Write the orbital notation for the • ↑↓ element ↑↓ ↑↓ selenium ↑↓ 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p

Write the Lewis Dot structure for the following atoms and their ions

Aluminum Al Al+3 (group 13; 3 valence electrons) (aluminum loses its 3 valence electrons to satisfy the octet rule)

Phosphorus P (group 15; 5 valence electrons) P -3 (phosphorus gains 3 electrons to satisfy the octet rule)

Helium He No (group 18 but it has 2 valence electrons) ionic form of helium. It already has a full valence shell so it already satisfies the octet rule

Strontium Sr Sr+2 (group 2; 2 valence electrons) (strontium loses its 2 valence electrons to satisfy the octet rule)
- Slides: 32