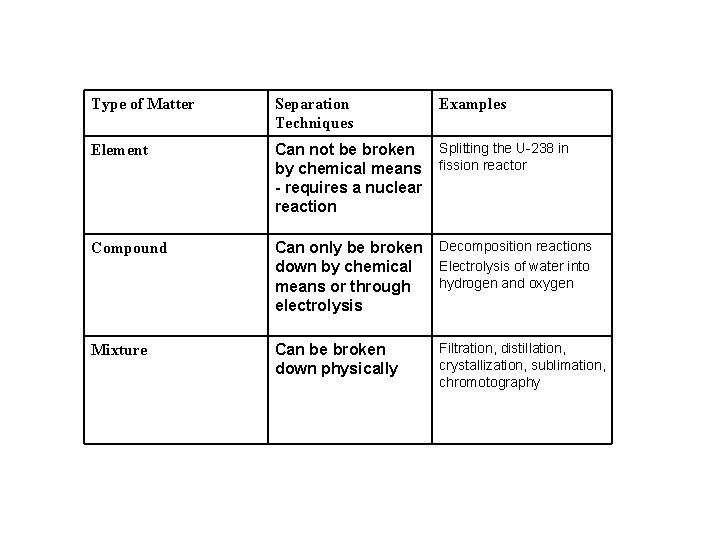
Type of Matter Separation Techniques Examples Element Can



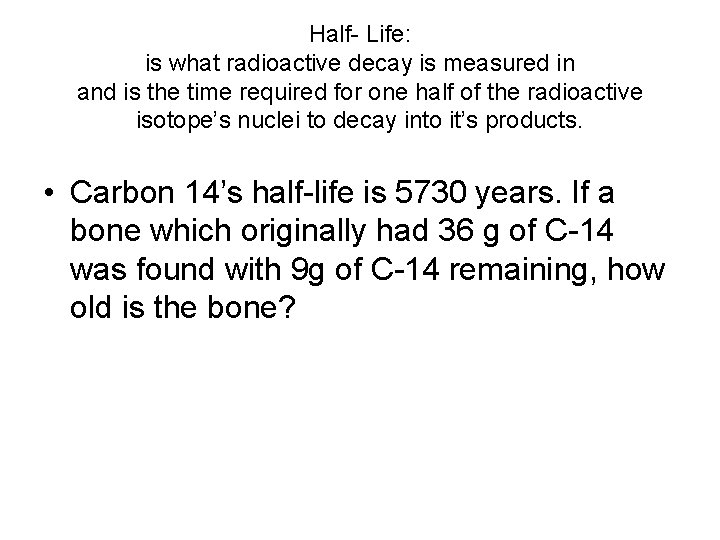

- Slides: 11

Type of Matter Separation Techniques Examples Element Can not be broken by chemical means - requires a nuclear reaction Splitting the U-238 in fission reactor Compound Can only be broken down by chemical means or through electrolysis Decomposition reactions Electrolysis of water into hydrogen and oxygen Mixture Can be broken down physically Filtration, distillation, crystallization, sublimation, chromotography

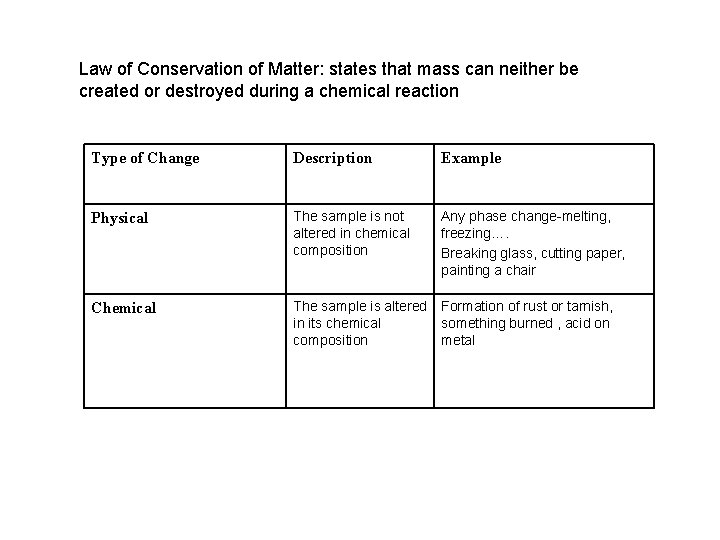
Law of Conservation of Matter: states that mass can neither be created or destroyed during a chemical reaction Type of Change Description Example Physical The sample is not altered in chemical composition Any phase change-melting, freezing…. Breaking glass, cutting paper, painting a chair Chemical The sample is altered Formation of rust or tarnish, in its chemical something burned , acid on composition metal

The History of Chemistry About 960 BCE- the Chinese monk Li Tian invented gunpowder (saltpeter- potassium nitrate, sulfur and charcoal) which also brought about the invention of fireworks Democritus (465 BCE) First to propose that matter exists in the form of particles. Coined the term 'atoms'. Aristotle (348 - 315 BCE) Established the subject of science in his school- thought matter was composed of earth, water, air and firethese were the 4 elements that made up everything- his knowledge was renowned and respected for over a 1000 years Alchemistry ( 1000 to 1650 CE) A group of individuals throughout the world that would try to convert common elements into gold 1100 CE- Evidence of a loadstone used in a compass Boyle, Sir Robert (1637 -1691) Formulated the fundamental gas laws. First to propose the combination of small particles to form molecules. Differentiated between compounds and mixtures. Priestley, Joseph (1733 -1804) Discovered oxygen, carbon monoxide, and nitrous oxide. Proposed electrical inverse-square law (1767). Lavoisier, A. L. (1743 -1794) Discovered nitrogen. Described the composition of many organic compounds. Sometimes regarded as the Father of

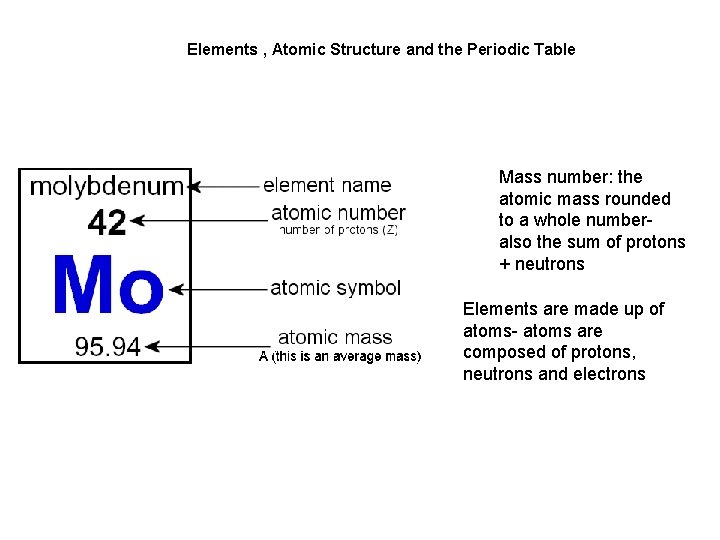
Elements , Atomic Structure and the Periodic Table Mass number: the atomic mass rounded to a whole numberalso the sum of protons + neutrons Elements are made up of atoms- atoms are composed of protons, neutrons and electrons

Avogadro, Amedeo (1776 -1856) Proposed principle that equal volumes of gases contain the same number of molecules. Gay-Lussac, J. L. (1778 -1850) Discovered boron and iodine. Discovered acid-base indicators (litmus). Improved method for making sulfuric acid. Researched behavior of gases. Mendeléev, Dmitri (1834 -1907) Discovered periodicity of the elements. Compiled the first Periodic Table with elements arranged into 7 groups (1869). Le Chatelier, H. L. (1850 -1936) Fundamental research on equilibrium reactions (Le Chatelier’s Law), combustion of gases, and iron and steel metallurgy. Thomson, Sir J. J. (1856 -1940) Research on cathode rays proved existence of electrons (1896). Nobel Prize in 1906 Rutherford, Sir Ernest (1871 -1937) Discovered that uranium radiation is composed of positively charged 'alpha' particles and negatively charged 'beta' particles (1989/1899). First to prove radioactive decay of heavy elements and to perform a transmutation reaction (1919). Discovered halflife of radioactive elements. Established that the nucleus was small, dense, and positively charged. Assumed that electrons were outside the nucleus. Nobel Prize in 1908.

Isotopes: Atoms of the same element with different number of neutronssome can be radioactive Calculating average atomic mass (weighted average): Boron B(10) 10. 012938 19. 80 B(11) 11. 009305 80. 20

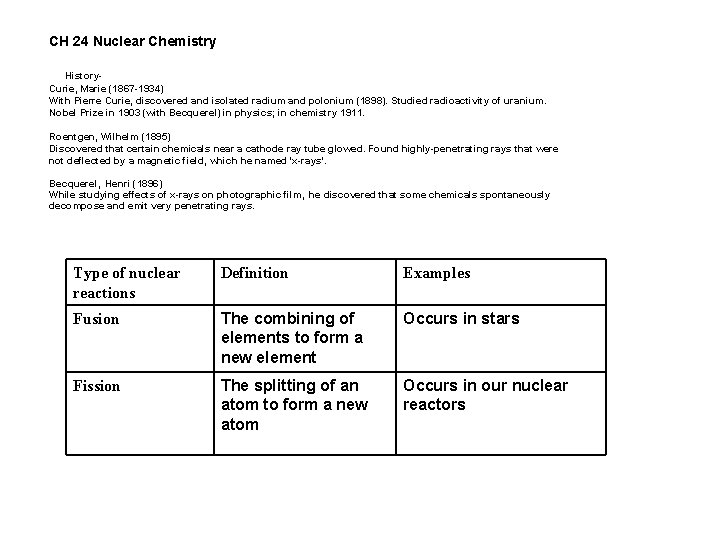
CH 24 Nuclear Chemistry History. Curie, Marie (1867 -1934) With Pierre Curie, discovered and isolated radium and polonium (1898). Studied radioactivity of uranium. Nobel Prize in 1903 (with Becquerel) in physics; in chemistry 1911. Roentgen, Wilhelm (1895) Discovered that certain chemicals near a cathode ray tube glowed. Found highly-penetrating rays that were not deflected by a magnetic field, which he named 'x-rays'. Becquerel, Henri (1896) While studying effects of x-rays on photographic film, he discovered that some chemicals spontaneously decompose and emit very penetrating rays. Type of nuclear reactions Definition Examples Fusion The combining of elements to form a new element Occurs in stars Fission The splitting of an atom to form a new atom Occurs in our nuclear reactors

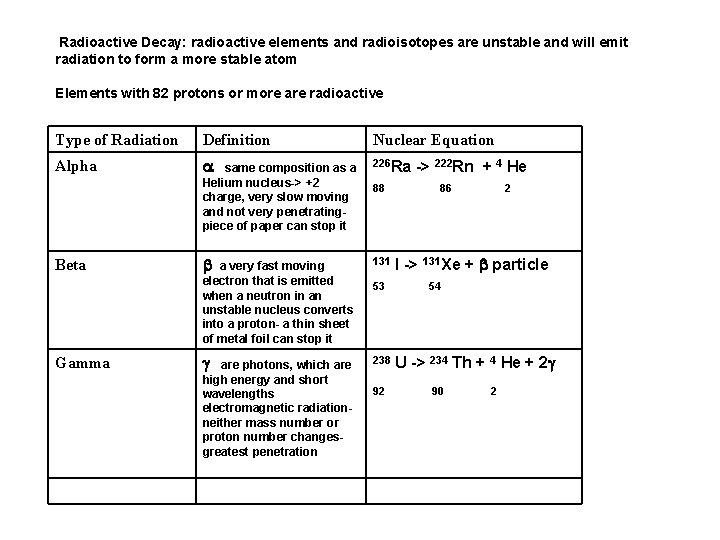
Radioactive Decay: radioactive elements and radioisotopes are unstable and will emit radiation to form a more stable atom Elements with 82 protons or more are radioactive Type of Radiation Definition Nuclear Equation Alpha a same composition as a Helium nucleus-> +2 charge, very slow moving and not very penetratingpiece of paper can stop it 226 Ra b a very fast moving electron that is emitted when a neutron in an unstable nucleus converts into a proton- a thin sheet of metal foil can stop it 131 g 238 Beta Gamma are photons, which are high energy and short wavelengths electromagnetic radiationneither mass number or proton number changesgreatest penetration 88 53 92 -> 222 Rn + 4 He 86 2 I -> 131 Xe + b particle 54 U -> 234 Th + 4 He + 2 g 90 2
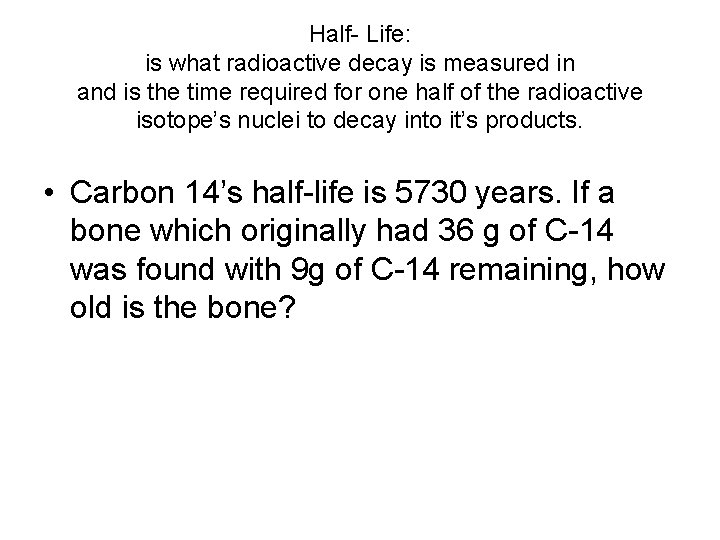
Half- Life: is what radioactive decay is measured in and is the time required for one half of the radioactive isotope’s nuclei to decay into it’s products. • Carbon 14’s half-life is 5730 years. If a bone which originally had 36 g of C-14 was found with 9 g of C-14 remaining, how old is the bone?

Uses and Concerns of nuclear reactionz 1) 2) 3) 4) 5) 6) Used as an energy source- nuclear reactors use fission Used in weapons Used in medicine for both diagnostic and to kill cancerous tumors Used to date objects Radioactive material never goes away it will always be here Radioactivity can cause cancer and death not just in humans but any living thing

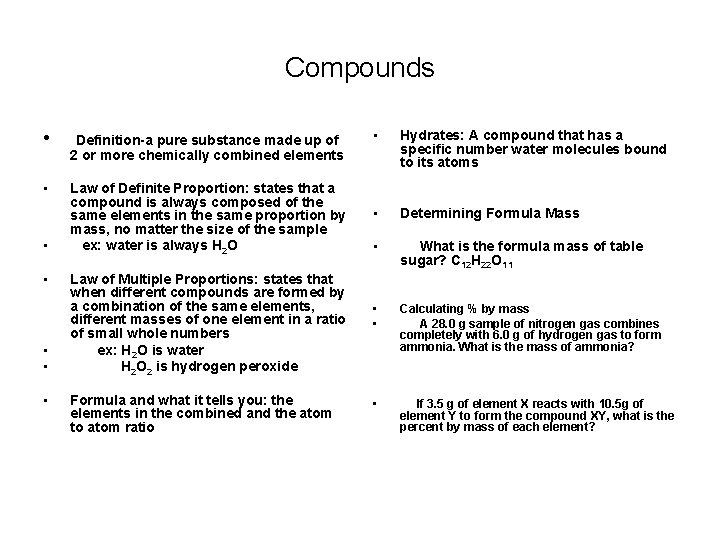
Compounds • Definition-a pure substance made up of 2 or more chemically combined elements • Law of Definite Proportion: states that a compound is always composed of the same elements in the same proportion by mass, no matter the size of the sample ex: water is always H 2 O • • • Law of Multiple Proportions: states that when different compounds are formed by a combination of the same elements, different masses of one element in a ratio of small whole numbers ex: H 2 O is water H 2 O 2 is hydrogen peroxide Formula and what it tells you: the elements in the combined and the atom to atom ratio • Hydrates: A compound that has a specific number water molecules bound to its atoms • Determining Formula Mass • What is the formula mass of table sugar? C 12 H 22 O 11 • • Calculating % by mass A 28. 0 g sample of nitrogen gas combines completely with 6. 0 g of hydrogen gas to form ammonia. What is the mass of ammonia? • If 3. 5 g of element X reacts with 10. 5 g of element Y to form the compound XY, what is the percent by mass of each element?