Type 2 Diabetes Mellitus treatment via SGLT 2

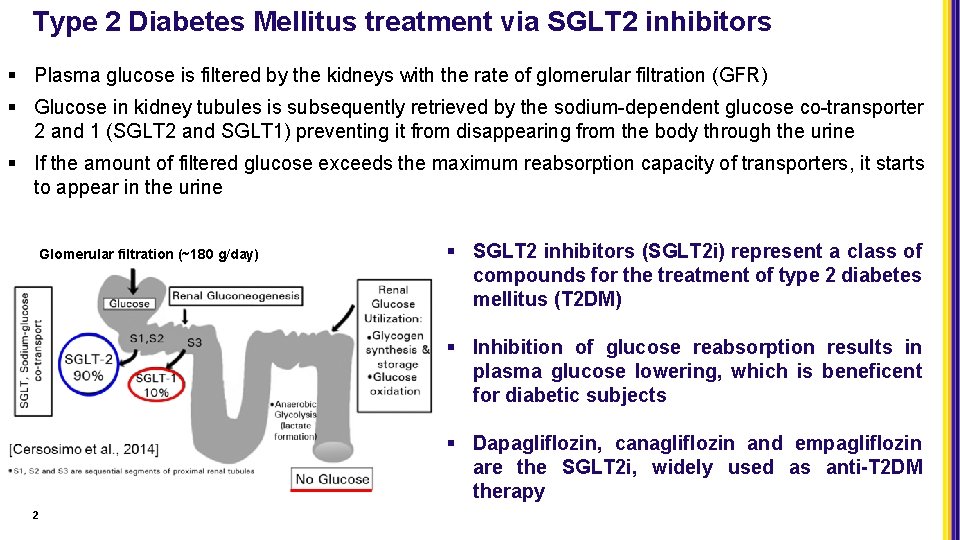
Type 2 Diabetes Mellitus treatment via SGLT 2 inhibitors § Plasma glucose is filtered by the kidneys with the rate of glomerular filtration (GFR) § Glucose in kidney tubules is subsequently retrieved by the sodium-dependent glucose co-transporter 2 and 1 (SGLT 2 and SGLT 1) preventing it from disappearing from the body through the urine § If the amount of filtered glucose exceeds the maximum reabsorption capacity of transporters, it starts to appear in the urine Glomerular filtration (~180 g/day) § SGLT 2 inhibitors (SGLT 2 i) represent a class of compounds for the treatment of type 2 diabetes mellitus (T 2 DM) § Inhibition of glucose reabsorption results in plasma glucose lowering, which is beneficent for diabetic subjects § Dapagliflozin, canagliflozin and empagliflozin are the SGLT 2 i, widely used as anti-T 2 DM therapy 2
Challenges in SGLT 2 i development § Application of dapagliflozin in type 1 diabetes mellitus (T 1 DM) in Japanese population § Greater efficacy of canagliflozin in T 2 DM population compared to other SGLT 2 i § Increased frequency of adverse events (bone fractures and amputations) for canagliflozin Pop. PKPD, quantitative systems pharmacology, meta-analysis 3
Exposure-response modeling comparison between T 1 DM Japanese and non-Japanese patients treated with dapagliflozin § Phase IIa study was performed to support the dapagliflozin approval in T 1 DM Japanese population. § No statistically significant differences were observed in 24 -hours urinary glucose excretion (UGE) between 5 and 10 mg dapagliflozin doses. ü Why 5 and 10 mg dapagliflozin cause the same 24 h UGE in Japanese study, but not in previously performed non-Japanese study? ü Should we adjust the optimal dose for Japanese population? 4 Population exposure response modeling using individual data from non-Japanese and Japanese studies
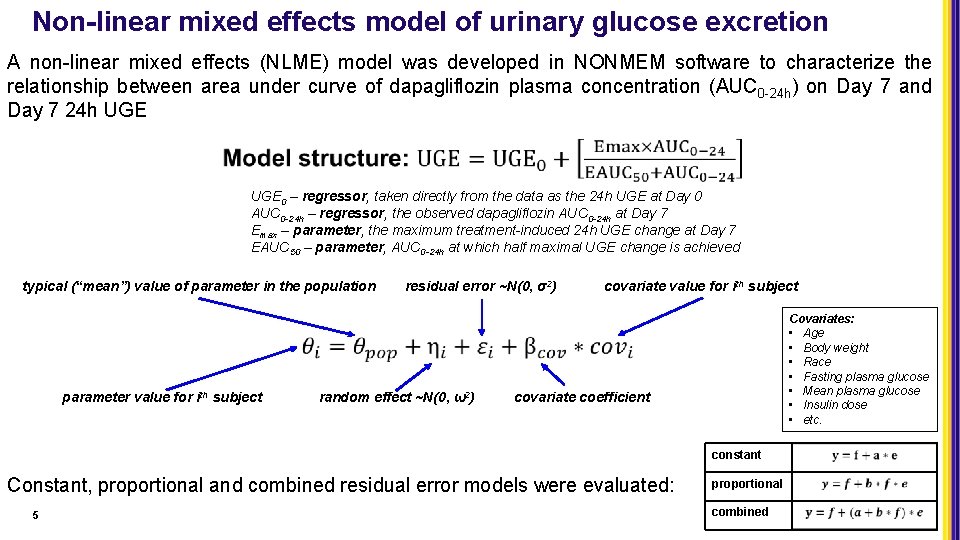
Non-linear mixed effects model of urinary glucose excretion A non-linear mixed effects (NLME) model was developed in NONMEM software to characterize the relationship between area under curve of dapagliflozin plasma concentration (AUC 0 -24 h) on Day 7 and Day 7 24 h UGE 0 – regressor, taken directly from the data as the 24 h UGE at Day 0 AUC 0 -24 h – regressor, the observed dapagliflozin AUC 0 -24 h at Day 7 Emax – parameter, the maximum treatment-induced 24 h UGE change at Day 7 EAUC 50 – parameter, AUC 0 -24 h at which half maximal UGE change is achieved typical (“mean”) value of parameter in the population parameter value for ith subject residual error ~N(0, σ2) random effect ~N(0, ω2) covariate value for ith subject Covariates: • Age • Body weight • Race • Fasting plasma glucose • Mean plasma glucose • Insulin dose • etc. covariate coefficient constant Constant, proportional and combined residual error models were evaluated: 5 proportional combined
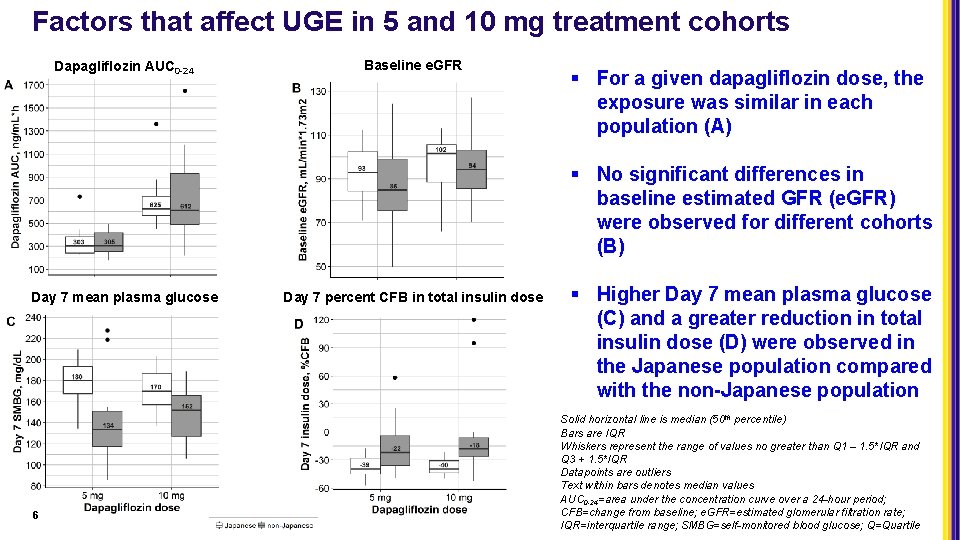
Factors that affect UGE in 5 and 10 mg treatment cohorts Dapagliflozin AUC 0 -24 Baseline e. GFR § For a given dapagliflozin dose, the exposure was similar in each population (A) § No significant differences in baseline estimated GFR (e. GFR) were observed for different cohorts (B) Day 7 mean plasma glucose 6 Day 7 percent CFB in total insulin dose § Higher Day 7 mean plasma glucose (C) and a greater reduction in total insulin dose (D) were observed in the Japanese population compared with the non-Japanese population Solid horizontal line is median (50 th percentile) Bars are IQR Whiskers represent the range of values no greater than Q 1 – 1. 5*IQR and Q 3 + 1. 5*IQR Datapoints are outliers Text within bars denotes median values AUC 0 -24=area under the concentration curve over a 24 -hour period; CFB=change from baseline; e. GFR=estimated glomerular filtration rate; IQR=interquartile range; SMBG=self-monitored blood glucose; Q=Quartile
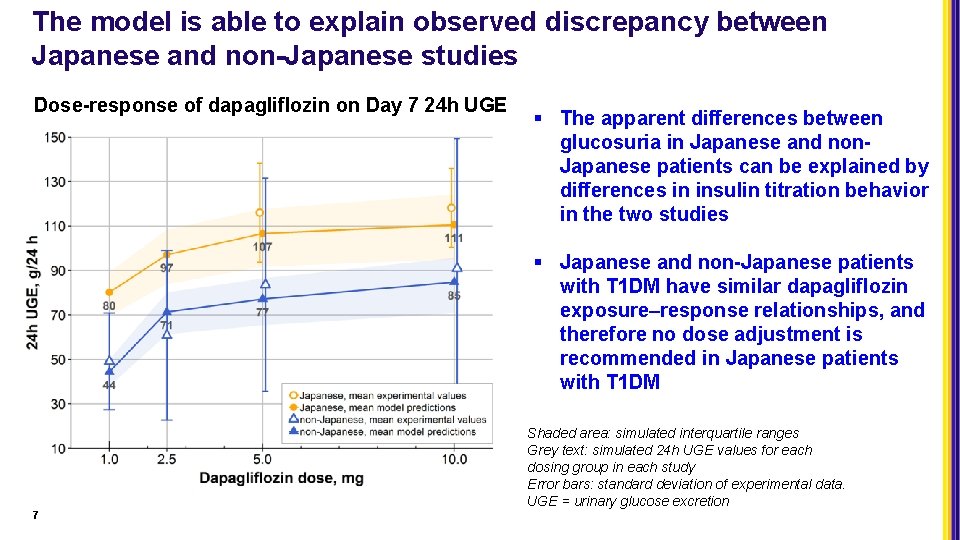
The model is able to explain observed discrepancy between Japanese and non-Japanese studies Dose-response of dapagliflozin on Day 7 24 h UGE § The apparent differences between glucosuria in Japanese and non. Japanese patients can be explained by differences in insulin titration behavior in the two studies § Japanese and non-Japanese patients with T 1 DM have similar dapagliflozin exposure–response relationships, and therefore no dose adjustment is recommended in Japanese patients with T 1 DM Shaded area: simulated interquartile ranges Grey text: simulated 24 h UGE values for each dosing group in each study Error bars: standard deviation of experimental data. UGE = urinary glucose excretion 7
Quantitative systems pharmacology (QSP) model of renal glucose reabsorption § Despite SGLT 2 contributing 80 -90% to renal glucose reabsorption, clinical observations showed SGLT 2 i decrease reabsorption by only 30– 50%. Canagliflozin, a dapagliflozin competitor, possesses higher efficacy in T 2 DM patients at marketed doses. § ü Why SGLT 2 inhibitors decrease reabsorption by 30– 50% only? ü Why canagliflozin shows greater UGE in subjects with T 2 DM? ü Are there any differences in reabsorption processes between healthy and T 2 DM subjects? A physiologically-based model of glucose filtration, reabsorption and excretion, that includes dapagliflozin, canagliflozin, empagliflozin 8
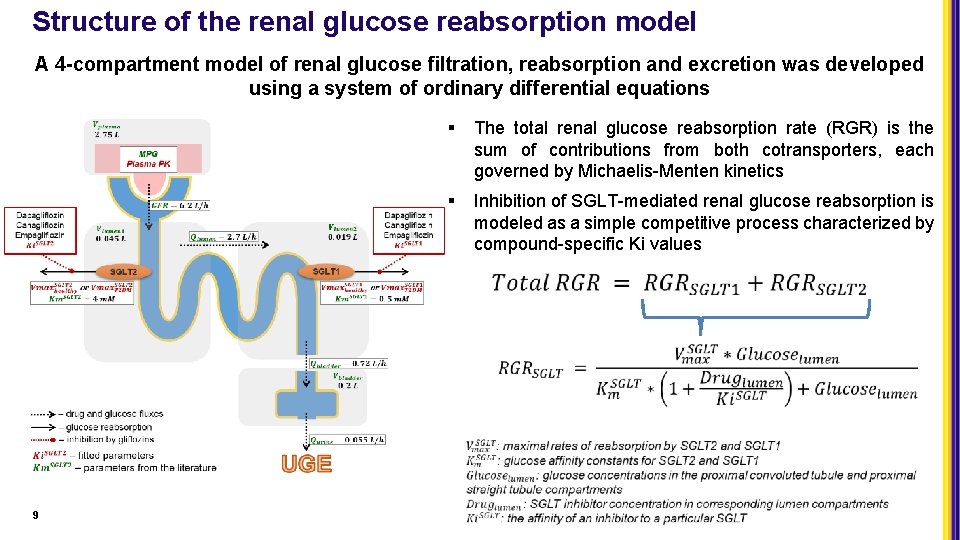
Structure of the renal glucose reabsorption model A 4 -compartment model of renal glucose filtration, reabsorption and excretion was developed using a system of ordinary differential equations 9 § The total renal glucose reabsorption rate (RGR) is the sum of contributions from both cotransporters, each governed by Michaelis-Menten kinetics § Inhibition of SGLT-mediated renal glucose reabsorption is modeled as a simple competitive process characterized by compound-specific Ki values
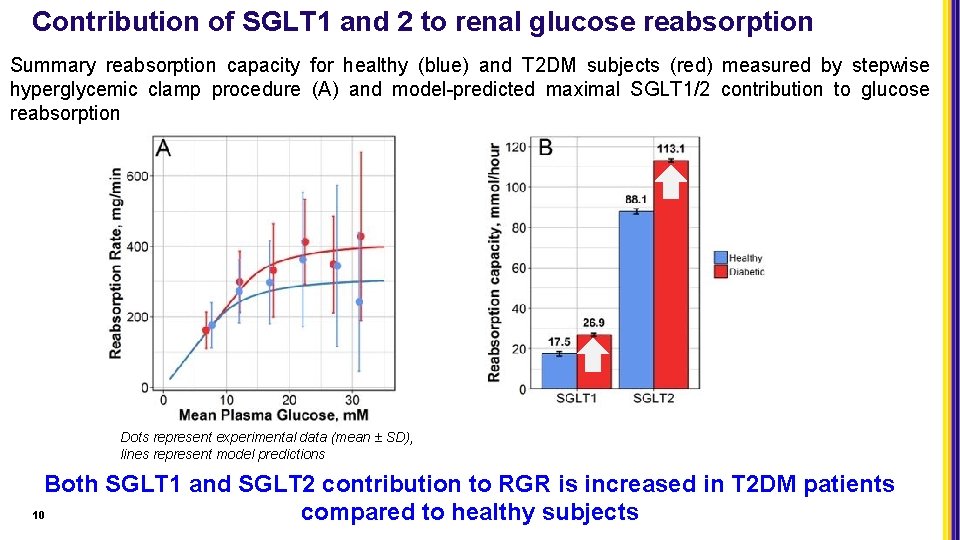
Contribution of SGLT 1 and 2 to renal glucose reabsorption Summary reabsorption capacity for healthy (blue) and T 2 DM subjects (red) measured by stepwise hyperglycemic clamp procedure (A) and model-predicted maximal SGLT 1/2 contribution to glucose reabsorption Dots represent experimental data (mean ± SD), lines represent model predictions Both SGLT 1 and SGLT 2 contribution to RGR is increased in T 2 DM patients 10 compared to healthy subjects
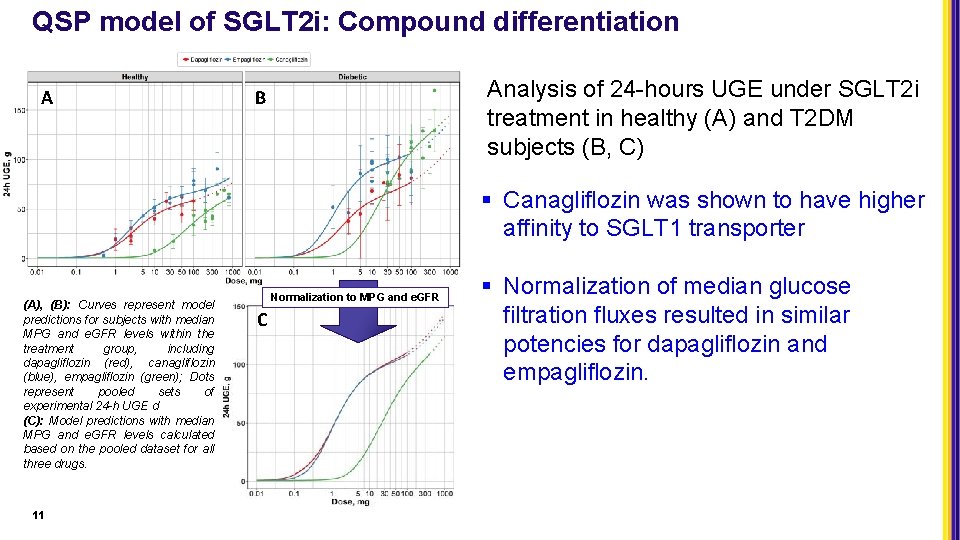
QSP model of SGLT 2 i: Compound differentiation A Analysis of 24 -hours UGE under SGLT 2 i treatment in healthy (A) and T 2 DM subjects (B, C) B § Canagliflozin was shown to have higher affinity to SGLT 1 transporter (A), (B): Curves represent model predictions for subjects with median MPG and e. GFR levels within the treatment group, including dapagliflozin (red), canagliflozin (blue), empagliflozin (green); Dots represent pooled sets of experimental 24 -h UGE d (C): Model predictions with median MPG and e. GFR levels calculated based on the pooled dataset for all three drugs. 11 Normalization to MPG and e. GFR C § Normalization of median glucose filtration fluxes resulted in similar potencies for dapagliflozin and empagliflozin.
Glucosuria-related adverse events for the SGLT 2 i dapagliflozin and canagliflozin: a meta-analysis § Canagliflozin was shown to increase the incidence of bone fractures and amputations ü Which adverse events are more frequent in canagliflozin-treated population compared to dapagliflozin? ü Is it a class effect for all SGLT 2 i or is it only canagliflozin-related? ü Is urinary glucose excretion a cause of these adverse events? Model-based meta-analysis of adverse evens in dapagliflozin- and canagliflozintreated populations 12
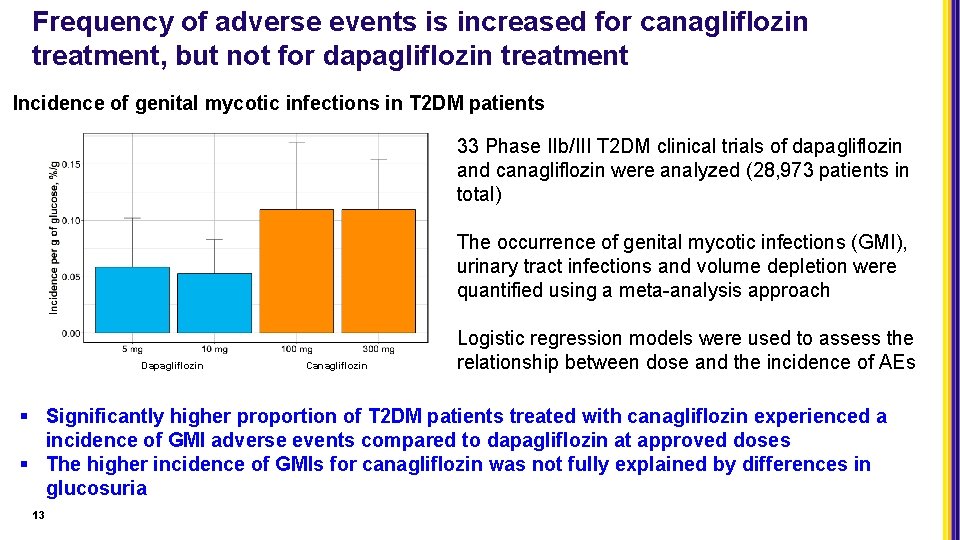
Frequency of adverse events is increased for canagliflozin treatment, but not for dapagliflozin treatment Incidence of genital mycotic infections in T 2 DM patients 33 Phase IIb/III T 2 DM clinical trials of dapagliflozin and canagliflozin were analyzed (28, 973 patients in total) The occurrence of genital mycotic infections (GMI), urinary tract infections and volume depletion were quantified using a meta-analysis approach Dapagliflozin Canagliflozin Logistic regression models were used to assess the relationship between dose and the incidence of AEs § Significantly higher proportion of T 2 DM patients treated with canagliflozin experienced a incidence of GMI adverse events compared to dapagliflozin at approved doses § The higher incidence of GMIs for canagliflozin was not fully explained by differences in glucosuria 13

Acknowledgments Victor Sokolov Kirill Peskov 14 Lulu Chu Weifeng Tang Peter J. Greasley Susanne Johansson Gabriel Helmlinger David W. Boulton Robert C. Penland Shinya Ueda Joanna Parkinson Sergey Aksenov Peter Greasley

Спасибо за внимание! Thank you for your attention!

Backup
Meta-analysis procedure • The Meta-analysis was performed using a random-effects model with the Maximum-Likelihood estimator. • Logit-transformed AEs were used. Certain AE subgroups of dapagliflozin, in all AE outcomes (male genital mycotic infections and volume depletion), were less than 5%, and are considered to be rare events. It has been shown that, for AE rates <5%, the normal distribution assumption for within-trial variability is no longer valid and leads to a bias in the estimation of the mean effect size [J Clin Epidemiol. 2008; 61: 41 -51] • For rare events as well as other events, we therefore used the normal-binomial general linear mixed model (GLMM) approach [Stat Med. 2010; 29: 3046 -67], as implemented in using the metafor package in R software (Version 3. 4. 4) • A risk difference between the treatment and placebo was then calculated to reveal the treatment effect for low doses and high doses. 17
- Slides: 17