Type 2 Diabetes Mellitus Treating to Target January

Type 2 Diabetes Mellitus Treating to Target January 22, 2004. Dr. William Harper Endocrinology & Metabolism Assisstant Professor of Medicine Mc. Master University www. drharper. ca

Complications Diabetes: Complications Macrovascular Stroke Microvascular Diabetic eye disease (retinopathy and cataracts) Heart disease and hypertension 2 -4 X increased risk Renal disease Peripheral vascular disease Erectile Dysfunction Peripheral Neuropathy Foot problems Meltzer et al. CMAJ 1998; 20(Suppl 8): S 1 -S 29.
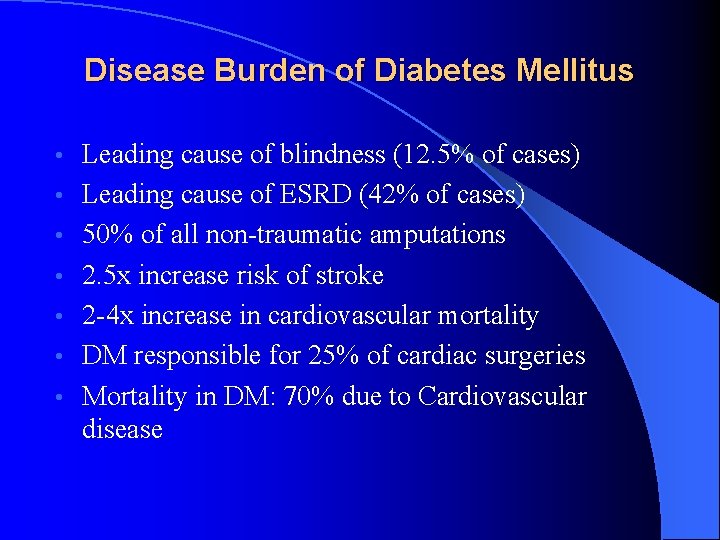
Disease Burden of Diabetes Mellitus • • Leading cause of blindness (12. 5% of cases) Leading cause of ESRD (42% of cases) 50% of all non-traumatic amputations 2. 5 x increase risk of stroke 2 -4 x increase in cardiovascular mortality DM responsible for 25% of cardiac surgeries Mortality in DM: 70% due to Cardiovascular disease
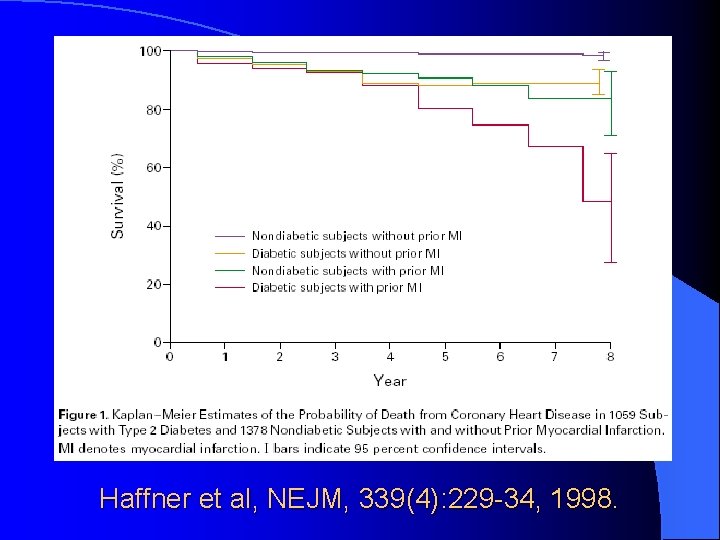
Haffner et al, NEJM, 339(4): 229 -34, 1998.
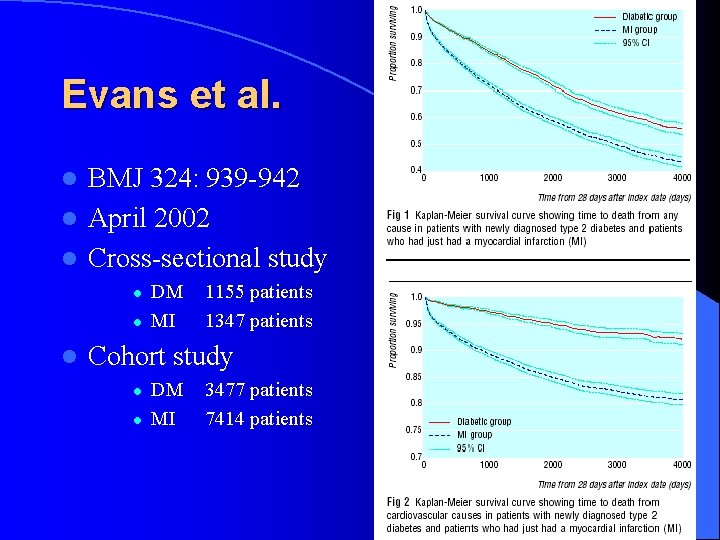
Evans et al. BMJ 324: 939 -942 l April 2002 l Cross-sectional study l l DM MI 1155 patients 1347 patients Cohort study l l DM MI 3477 patients 7414 patients
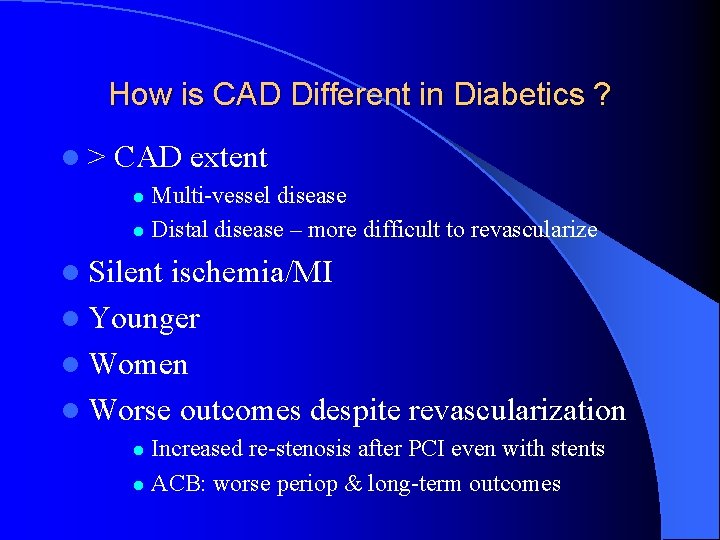
How is CAD Different in Diabetics ? l> CAD extent Multi-vessel disease l Distal disease – more difficult to revascularize l l Silent ischemia/MI l Younger l Women l Worse outcomes despite revascularization Increased re-stenosis after PCI even with stents l ACB: worse periop & long-term outcomes l

T 2 DM: “Rx to Targets” What are the targets?
What are the targets? l Cardiovascular risk factor modification ASA, Smoking Cessation l Lipids l Blood Pressure l • Proteinuria/DM nephropathy • Angiotensin II attenuation benefits independent of BP l Glycemic control Microvascular benefit l Macrovascular benefit ? l Target insulin resistance > insulin deficiency ? l
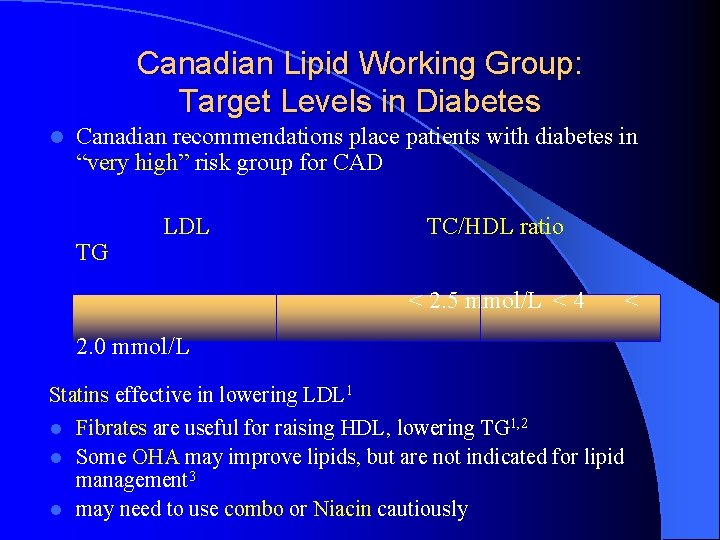
Canadian Lipid Working Group: Target Levels in Diabetes l Canadian recommendations place patients with diabetes in “very high” risk group for CAD TG LDL TC/HDL ratio < 2. 5 mmol/L < 4 2. 0 mmol/L Statins effective in lowering LDL 1 l Fibrates are useful for raising HDL, lowering TG 1, 2 l Some OHA may improve lipids, but are not indicated for lipid management 3 l may need to use combo or Niacin cautiously <
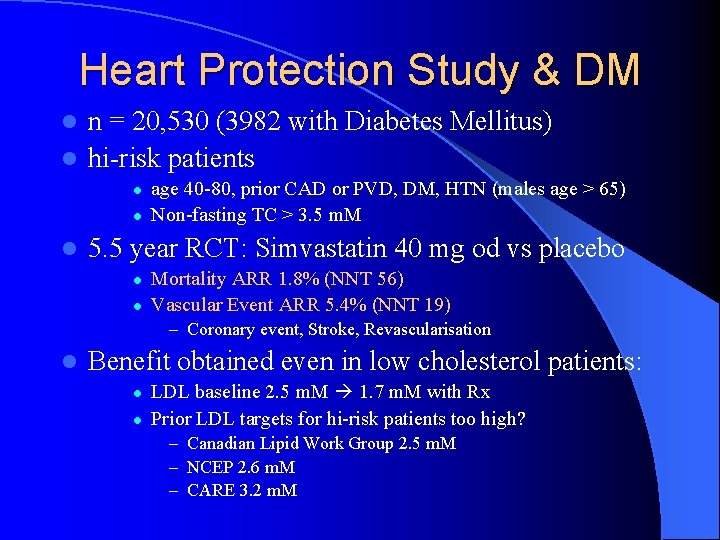
Heart Protection Study & DM n = 20, 530 (3982 with Diabetes Mellitus) l hi-risk patients l l age 40 -80, prior CAD or PVD, DM, HTN (males age > 65) Non-fasting TC > 3. 5 m. M 5. 5 year RCT: Simvastatin 40 mg od vs placebo l l Mortality ARR 1. 8% (NNT 56) Vascular Event ARR 5. 4% (NNT 19) – Coronary event, Stroke, Revascularisation l Benefit obtained even in low cholesterol patients: l l LDL baseline 2. 5 m. M 1. 7 m. M with Rx Prior LDL targets for hi-risk patients too high? – Canadian Lipid Work Group 2. 5 m. M – NCEP 2. 6 m. M – CARE 3. 2 m. M
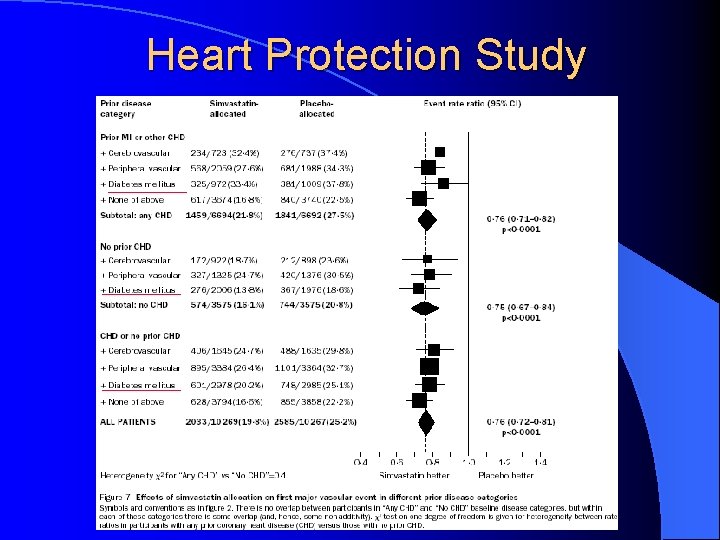
Heart Protection Study
Lipids & DM l What about HDL & TG? l Fibrates > Statins at HDL and TG l VA-HIT, a “low HDL Study” 2531 patients (620 DM), hi-risk with prior CAD l HDL < 1. 0 m. M, TG < 3. 4 m. M, LDL < 3. 6 m. M l RCT Gemfibrozil 600 mg po bid l Coronary death or MI ARR 4. 4% (NNT 23) l LDL 2. 3 -3. 6 m. M at baseline l Not on a statin despite LDL > 2. 5 m. M l

Lipids & DM l In DM patients where LDL is already adequately controlled by a statin, will the addition of a fibrate provide further benefit? l ACCORD: 10, 000 patients with ½ Lipid control arm l RCT: simvastatin + fenofibrate v. s. placebo l Results… l
What are the targets? l Cardiovascular risk factor modification ASA, Smoking Cessation l Lipids l Blood Pressure l • Proteinuria/DM nephropathy • Angiotensin II attenuation benefits independent of BP l Glycemic control Microvascular benefit l Macrovascular benefit ? l Target insulin resistance > insulin deficiency ? l
DM: BP cntrl l Difficult to consider BP cntrl in DM without also taking into account: Proteinuria/DM nephropathy l Cardiovascular benefit of reducing angiotensin II action independent of BP l
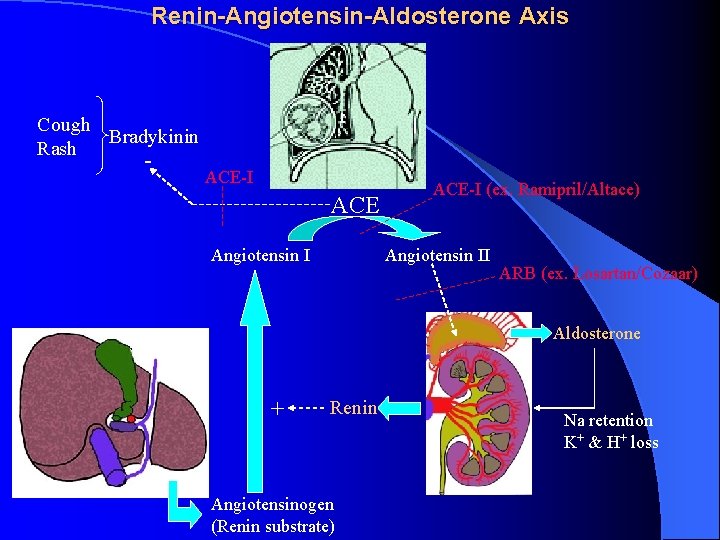
Renin-Angiotensin-Aldosterone Axis Cough Bradykinin Rash - ACE-I ACE Angiotensin I ACE-I (ex. Ramipril/Altace) Angiotensin II ARB (ex. Losartan/Cozaar) Aldosterone + Renin Angiotensinogen (Renin substrate) Na retention K+ & H+ loss

BP Trials in DM patients (some) l UKPDS l HOT l ALLHAT l LIFE l HOPE
BP Trials in DM patients UKPDS l atenolol = captopril at reducing outomes (UKPDS 39) l Benefit to reducing SBP < 120 (UKPDS 36, post-hoc subgroup analysis) l Currently SBP target < 120 being assessed in BP arm of the ACCORD Study l
BP Trials in DM patients UKPDS: atenolol = captopril in events l HOT: felodipine, CV events with DBP < 80 l ALLHAT l l LIFE (DM substudy) l l l Chlorthalidone > lisinopril or amlodipine (less CHF) Chlorthalidone BS/diagnosis of DM 1195 patients with DM/HTN/LVH Losartan > atenolol in CV death/MI/CVA despite equivalent BP lowering effects HOPE: not a BP trial per se
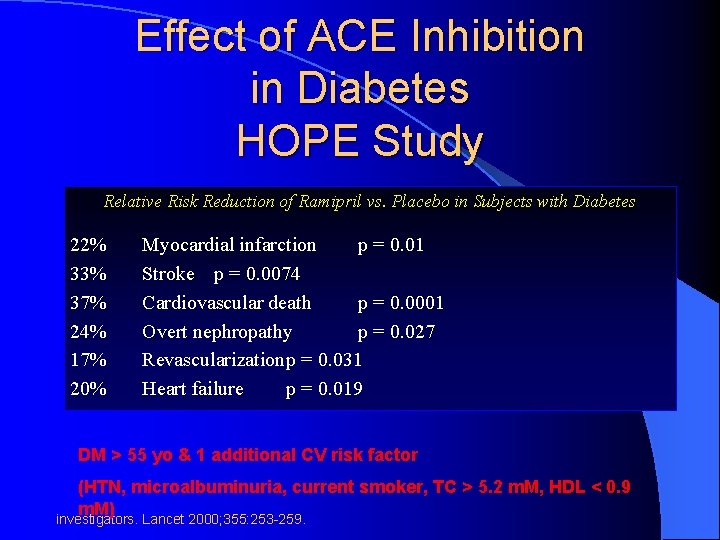
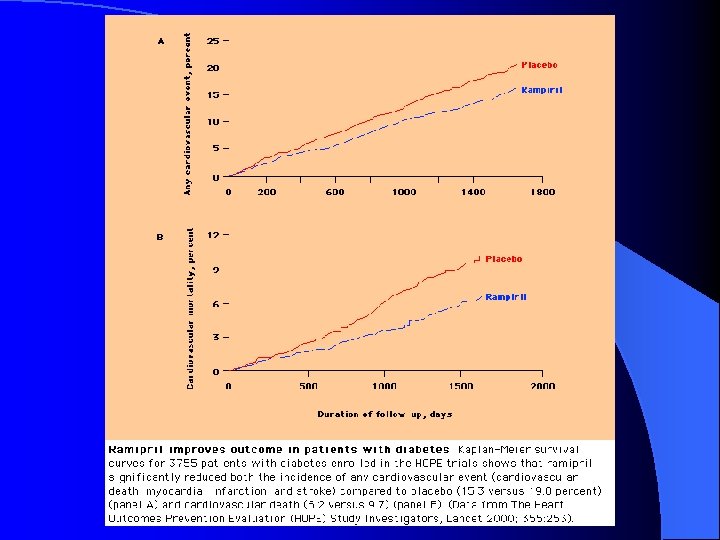
Complications Effect of ACE Inhibition in Diabetes HOPE Study Relative Risk Reduction of Ramipril vs. Placebo in Subjects with Diabetes 22% 33% 37% 24% 17% 20% Myocardial infarction p = 0. 01 Stroke p = 0. 0074 Cardiovascular death p = 0. 0001 Overt nephropathy p = 0. 027 Revascularizationp = 0. 031 Heart failure p = 0. 019 DM > 55 yo & 1 additional CV risk factor (HTN, microalbuminuria, current smoker, TC > 5. 2 m. M, HDL < 0. 9 m. M) HOPE investigators. Lancet 2000; 355: 253 -259.
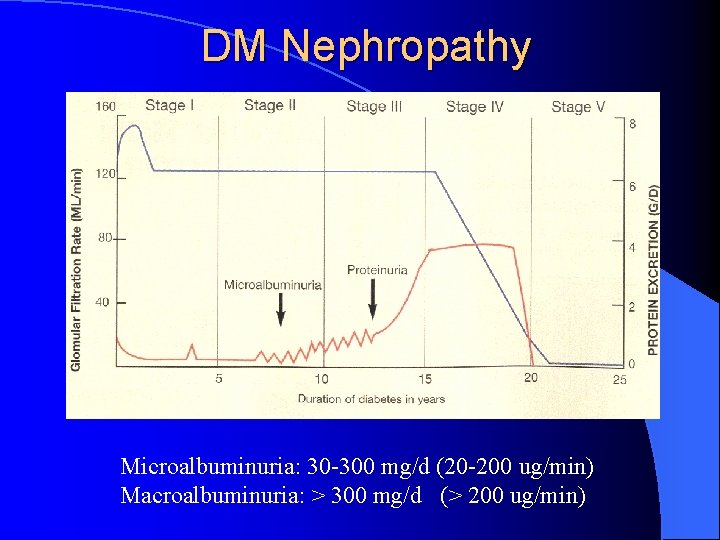
DM Nephropathy Microalbuminuria: 30 -300 mg/d (20 -200 ug/min) Macroalbuminuria: > 300 mg/d (> 200 ug/min)

DM Nephropathy & BP l T 1 DM: ACE-I albumin excretion and progression to overt nephropathy (macroalbuminuria) in patients with microalbuminuria even if BP is normal l ACE-I progression to ESRD or death in patients with overt nephropathy and serum creat > 132 u. M l
DM Nephropathy & BP l T 2 DM: l l l ACE-I & ARB progression of micro to macro albuminuria In contrast to T 1 DM, no demonstrated benefit of ACE-I over other anti-HTN (ex. UKPDS 39 captopril vs atenolol) in preventing ESRD in patients with overt nephropathy ARBs have been shown to be renal protective in T 2 DM with overt nephropathy: • IDNT: irbesartan (Avapro) ESRD/death/creat 2 x • RENAAL: losartan (Cozaar) creat 2 x and ESRD • Note: in both IDNT and RENAAL all ACE-I were stopped during the study
ACE-I & ARB Combination? STENO-2: part of a multifactorial approach (only 28% patients) l CHF Studies: l l CHARM: candesartan (Atacand) mortality, CHF admits, and onset of DM Val-He. FT: valsartan (Diovan) CHF admits CALM Study: l l l Mogensen et. al. BMJ 2000; 321: 1440 -1444 T 2 DM, HTN, microalbuminuria candesartan & lisinopril (Zestril, Prinivil) 12 wk study outcomes: BP, proteinuria
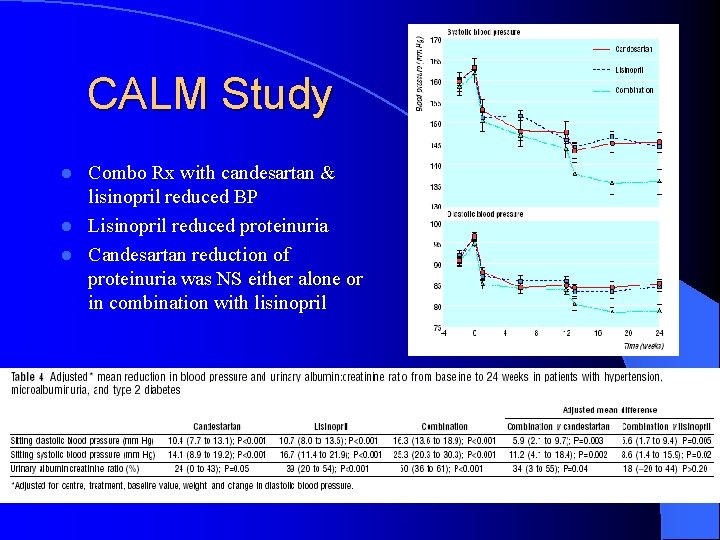
CALM Study Combo Rx with candesartan & lisinopril reduced BP l Lisinopril reduced proteinuria l Candesartan reduction of proteinuria was NS either alone or in combination with lisinopril l

BP Cntrl in DM: CHEP guidelines Canadian Hypertension Education Program l BP target: l l l < 130/80 (SBP 120? (ACCORD) / HOT target DBP 80) < 125/75 Proteinuria > 1 gm/d Initial therapy 2 nd line therapy DM with nephropathy ACE-I or ARB Addition of 1 or more of thiazide, -blocker, CCB, or an ACE-I/ARB combo DM without nephropathy ACE-I, ARB, or thiazide Combo of 1 st line drugs or addition of -blocker and/or CCB
What are the targets? l Cardiovascular risk factor modification ASA, Smoking Cessation l Lipids l Blood Pressure l • Proteinuria/DM nephropathy • Angiotensin II attenuation benefits independent of BP l Glycemic control Microvascular benefit l Macrovascular benefit ? l Target insulin resistance > insulin deficiency ? l

Glycemic Control l UKPDS 33, Lancet 352: 837 -53, 1998. l RCT of a policy of intensive BS control FPG < 6 m. M v. s. FPG < 15 m. M l Achieved a number of ways: l – Sulfonylurea (chlorpropamide or glibenclamide/glyburide) – Metformin (overweight subgroup, add-on) – Insulin (bedtime basal +/- basal/bolus regimens)
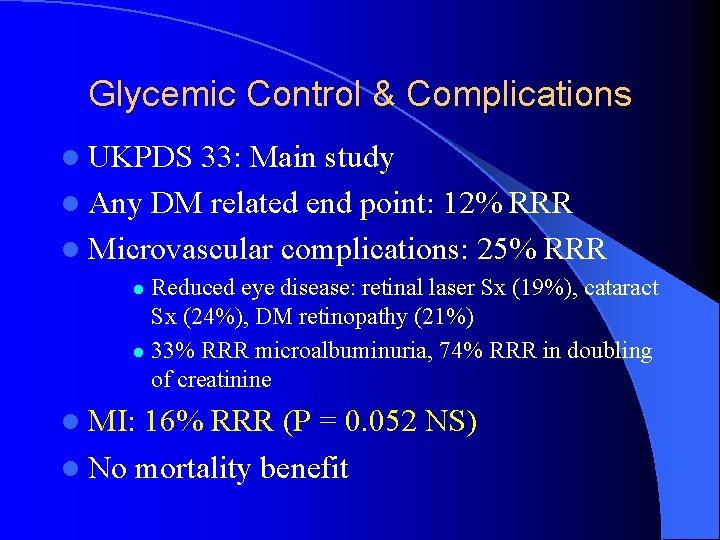
Glycemic Control & Complications l UKPDS 33: Main study l Any DM related end point: 12% RRR l Microvascular complications: 25% RRR Reduced eye disease: retinal laser Sx (19%), cataract Sx (24%), DM retinopathy (21%) l 33% RRR microalbuminuria, 74% RRR in doubling of creatinine l l MI: 16% RRR (P = 0. 052 NS) l No mortality benefit
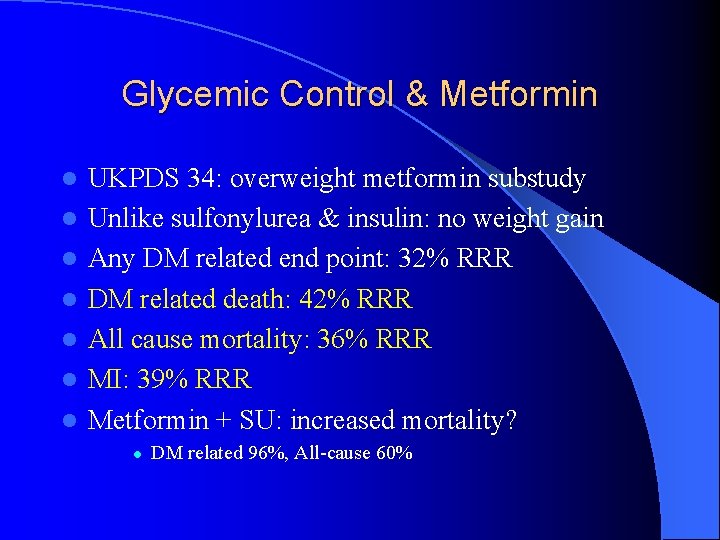
Glycemic Control & Metformin l l l l UKPDS 34: overweight metformin substudy Unlike sulfonylurea & insulin: no weight gain Any DM related end point: 32% RRR DM related death: 42% RRR All cause mortality: 36% RRR MI: 39% RRR Metformin + SU: increased mortality? l DM related 96%, All-cause 60%

T 2 DM & Macrovascular disease Why no clear benefit in UKPDS to glycemic cntrl? l Low CV risk patients: l l UKPDS cntrl death rate: 1. 2 % per year HOPE cntrl death rate: per year 2. 5% per year Unable to maintain glycemic cntrl due to limited interventions: l l l Available: glyburide, chlorpropamide, metformin, regular insulin No newer sulfonylureas: glimepiride (Amaryl), gliclazide (diamicron) No meglitinides: repaglinide (Gluconorm), nateglinide (Starlix) No TZD’s: rosiglitazone (Avandia), pioglitazone (Actos) No insulin analogues: (Humalog, Novorapid, Lantus)
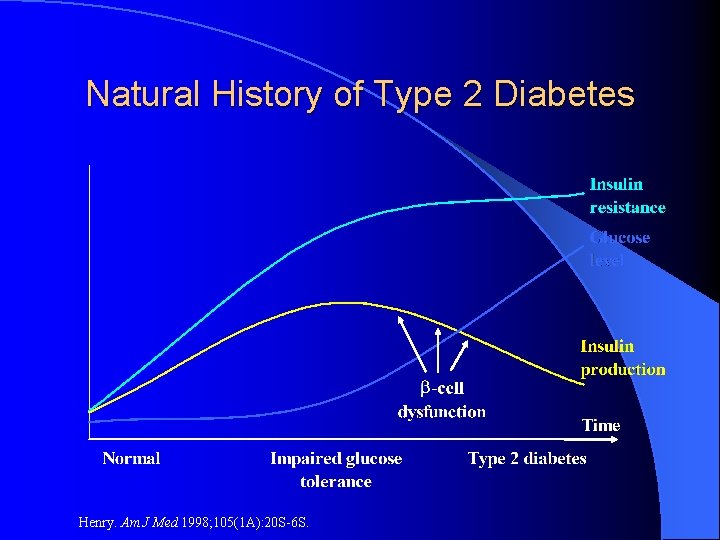
Natural History of Type 2 Diabetes Henry. Am J Med 1998; 105(1 A): 20 S-6 S.
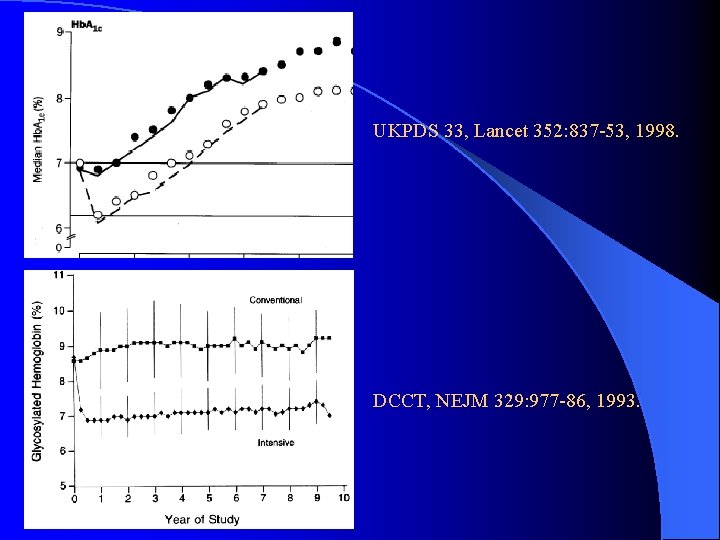
UKPDS 33, Lancet 352: 837 -53, 1998. DCCT, NEJM 329: 977 -86, 1993.
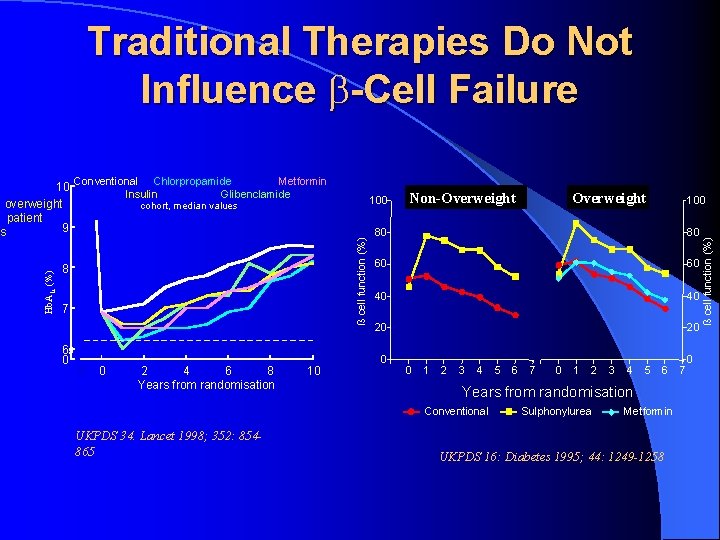
Traditional Therapies Do Not Influence -Cell Failure 8 7 6 0 100 cohort, median values -1 0 2 4 6 8 Years from randomisation 10 Non-Overweight Non obese 100 80 80 60 60 40 40 20 20 0 0 1 2 3 4 5 6 7 0 1 2 3 4 5 6 Years from randomisation Conventional UKPDS 34. Lancet 1998; 352: 854865 Overweight Obese Sulphonylurea Metformin UKPDS 16: Diabetes 1995; 44: 1249 -1258 7 0 ß cell function (%) Conventional Chlorpropamide Metformin Insulin Glibenclamide ß cell function (%) Hb. A 1 c (%) 10 overweight patient 9 s
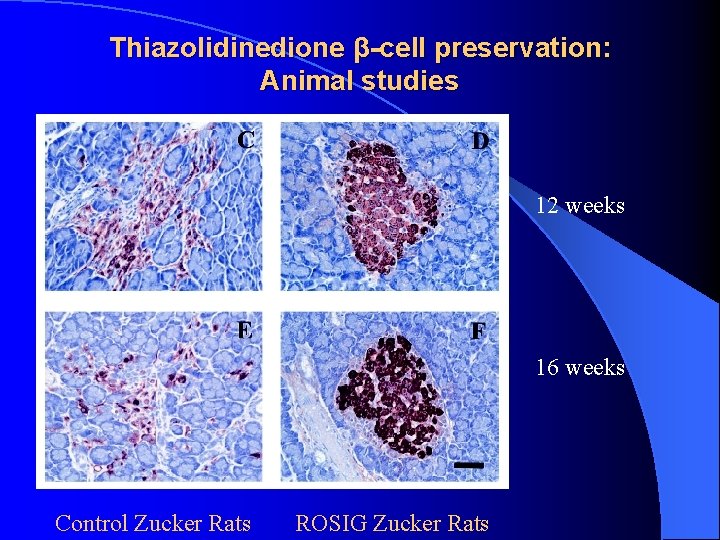
Thiazolidinedione β-cell preservation: Animal studies 12 weeks 16 weeks Control Zucker Rats ROSIG Zucker Rats
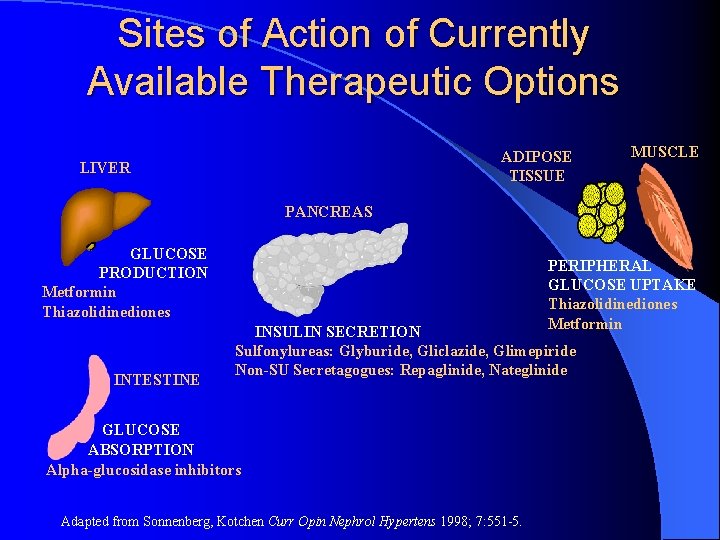
Sites of Action of Currently Available Therapeutic Options ADIPOSE TISSUE LIVER MUSCLE PANCREAS GLUCOSE PRODUCTION Metformin Thiazolidinediones INTESTINE PERIPHERAL GLUCOSE UPTAKE Thiazolidinediones Metformin INSULIN SECRETION Sulfonylureas: Glyburide, Gliclazide, Glimepiride Non-SU Secretagogues: Repaglinide, Nateglinide GLUCOSE ABSORPTION Alpha-glucosidase inhibitors Adapted from Sonnenberg, Kotchen Curr Opin Nephrol Hypertens 1998; 7: 551 -5.
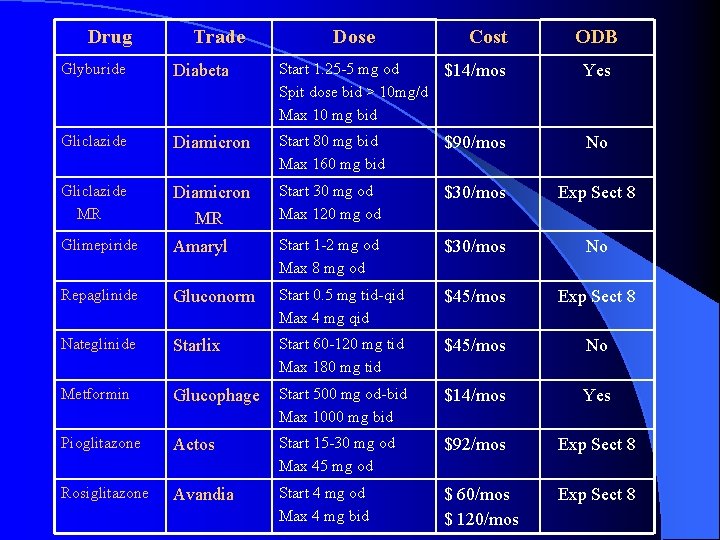
Drug Trade Dose Cost ODB Glyburide Diabeta Start 1. 25 -5 mg od $14/mos Spit dose bid > 10 mg/d Max 10 mg bid Yes Gliclazide Diamicron Start 80 mg bid Max 160 mg bid $90/mos No Gliclazide MR Diamicron MR Start 30 mg od Max 120 mg od $30/mos Exp Sect 8 Glimepiride Amaryl Start 1 -2 mg od Max 8 mg od $30/mos No Repaglinide Gluconorm Start 0. 5 mg tid-qid Max 4 mg qid $45/mos Exp Sect 8 Nateglinide Starlix Start 60 -120 mg tid Max 180 mg tid $45/mos No Metformin Glucophage Start 500 mg od-bid Max 1000 mg bid $14/mos Yes Pioglitazone Actos Start 15 -30 mg od Max 45 mg od $92/mos Exp Sect 8 Rosiglitazone Avandia Start 4 mg od Max 4 mg bid $ 60/mos $ 120/mos Exp Sect 8
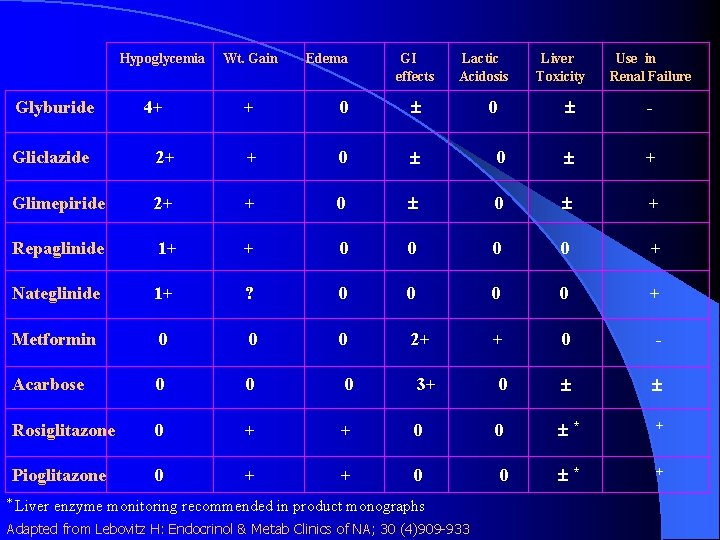
Hypoglycemia Glyburide 4+ Wt. Gain Edema GI effects + 0 Lactic Acidosis 0 Liver Toxicity Use in Renal Failure - Gliclazide 2+ + 0 0 + Glimepiride 2+ + 0 0 + Repaglinide 1+ + 0 0 + Nateglinide 1+ ? 0 0 + Metformin 0 0 0 2+ + 0 - Acarbose 0 0 0 3+ 0 Rosiglitazone 0 + + 0 0 * + Pioglitazone 0 + + 0 0 * + * Liver enzyme monitoring recommended in product monographs Adapted from Lebovitz H: Endocrinol & Metab Clinics of NA; 30 (4)909 -933
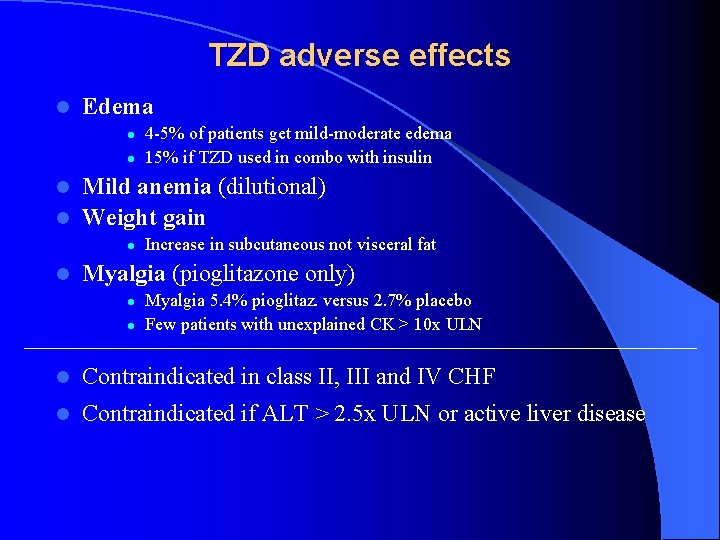
TZD adverse effects l Edema l l 4 -5% of patients get mild-moderate edema 15% if TZD used in combo with insulin Mild anemia (dilutional) l Weight gain l l l Increase in subcutaneous not visceral fat Myalgia (pioglitazone only) l l Myalgia 5. 4% pioglitaz. versus 2. 7% placebo Few patients with unexplained CK > 10 x ULN l Contraindicated in class II, III and IV CHF l Contraindicated if ALT > 2. 5 x ULN or active liver disease
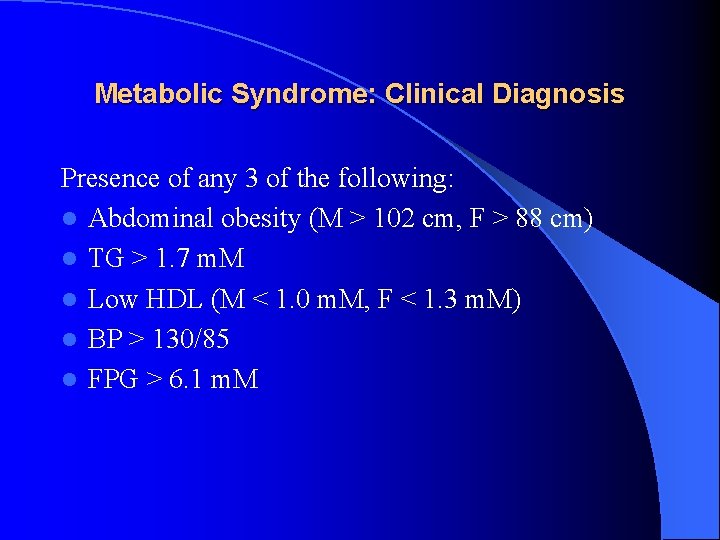
Metabolic Syndrome: Clinical Diagnosis Presence of any 3 of the following: l Abdominal obesity (M > 102 cm, F > 88 cm) l TG > 1. 7 m. M l Low HDL (M < 1. 0 m. M, F < 1. 3 m. M) l BP > 130/85 l FPG > 6. 1 m. M
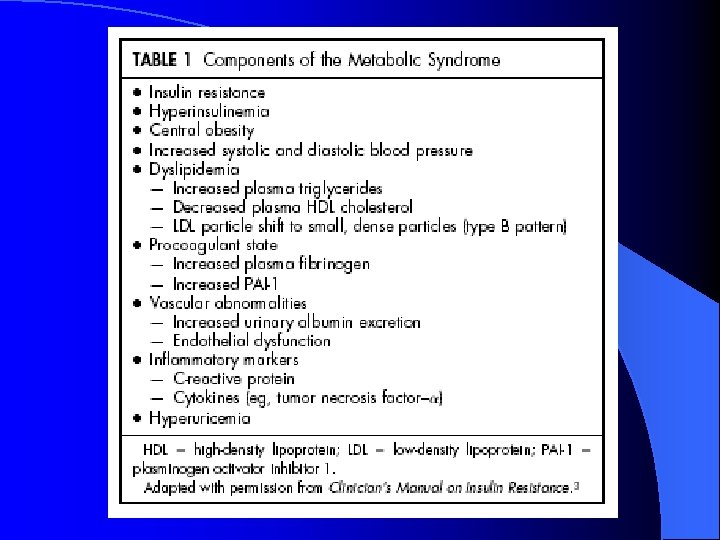
TZDs: effect on Metabolic Syndrome Reduce insulin resistance/blood sugar l Mild decrease in diastolic BP (2 -4 mm. Hg) l Decrease PAI-1 (reduces procoagulant state) l Lipids: l – ↓TG ↑HDL (pioglitazone > rosiglitazone? ) – ↓LDL (pioglitazone) – ↑LDL (rosiglitazone) l No change in Apo. B so ↑ due to larger less atherogenic particle size l Decrease in carotid artery intimal-media thickness (IMT)

Targeting Insulin Resistance? Does targeting insulin resistance > insulin secretion reduce CV risk? l We don’t know yet! l BARI-2 D: l l CV outomes Insulin sparing regimen (avandia, metformin) versus Insulin providing regimen (sulfonylurea, insulin) PPAR, RECORD, PROACTIVE l TZD’s, CV outcomes

Targeting insulin Secretion? Improve glycemic control in hi-risk patients to reduce CV risk l Using novel agents to get there! l ACCORD – glycemic cntrl arm Hb. A 1 c < 6 % l l glimepiride, insulin glargine, (and rosiglitazone) NAVIGATOR – nateglinide l DIGAMI II - insulin l ORIGIN, STREAM – insulin glargine l
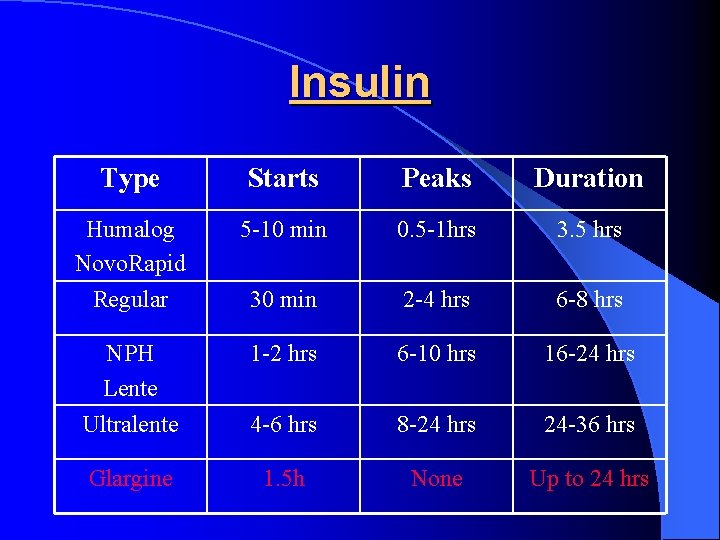
Insulin Type Starts Peaks Duration Humalog Novo. Rapid 5 -10 min 0. 5 -1 hrs 3. 5 hrs Regular 30 min 2 -4 hrs 6 -8 hrs NPH Lente Ultralente 1 -2 hrs 6 -10 hrs 16 -24 hrs 4 -6 hrs 8 -24 hrs 24 -36 hrs Glargine 1. 5 h None Up to 24 hrs

Insulin Glargine (Lantus) Substitution of glycine and arginine residues gives name “glargine” l 2 arginine residues make glargine more soluble in acidic p. H of injection medium but less soluble in physilogic p. H of sub. Q tissues l Once injected, glargine precipitates leading to slower absorption l Glycine substitution prevents degradation in sub. Q tissues l
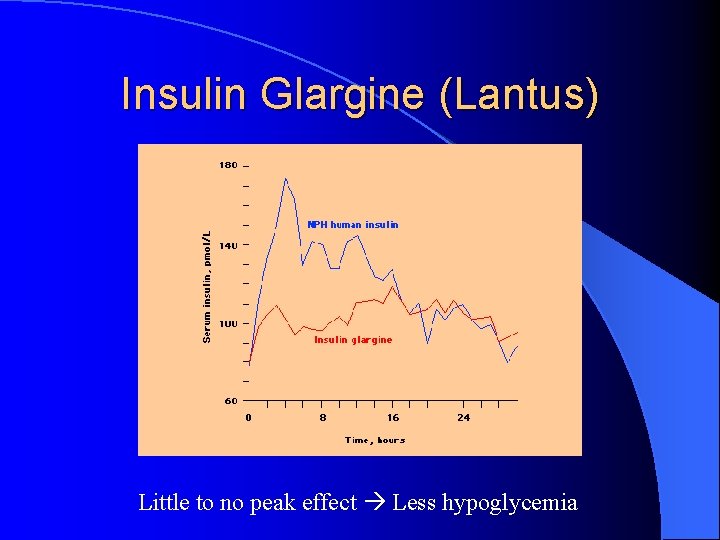
Insulin Glargine (Lantus) Little to no peak effect Less hypoglycemia
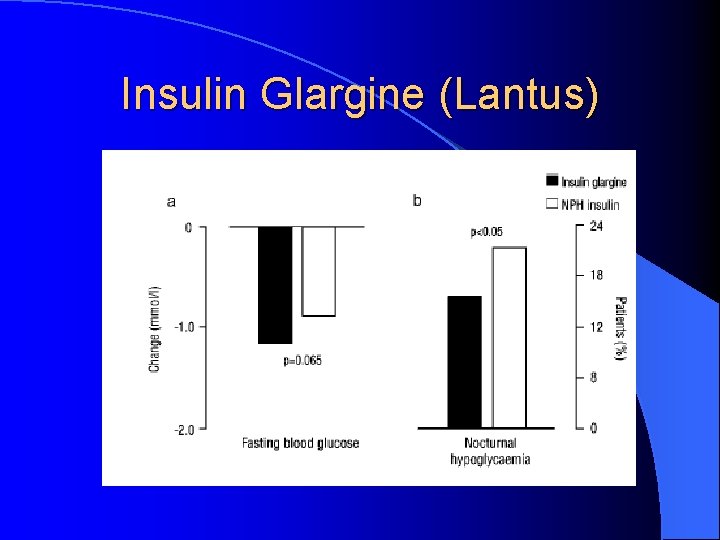
Insulin Glargine (Lantus)

BIDS Therapy l T 2 DM: “Introduction to insulin” l Keep on OHAs l Start 0. 2 U/kg SC qhs NPH or Lantus l Increase by 2 -4 U q 4 d until FBS 4 -7

Putting it all together l Any evidence that a multifactorial approach (targeting glycemic cntrl, BP, lipids, proteinuria, etc. ) works?

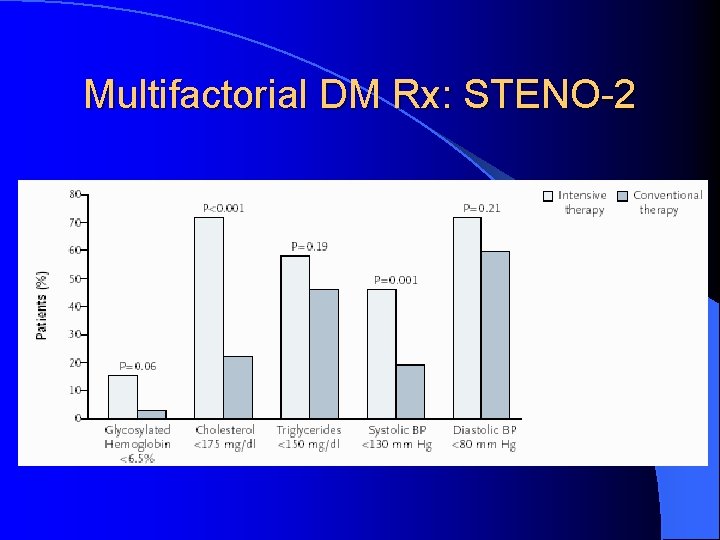
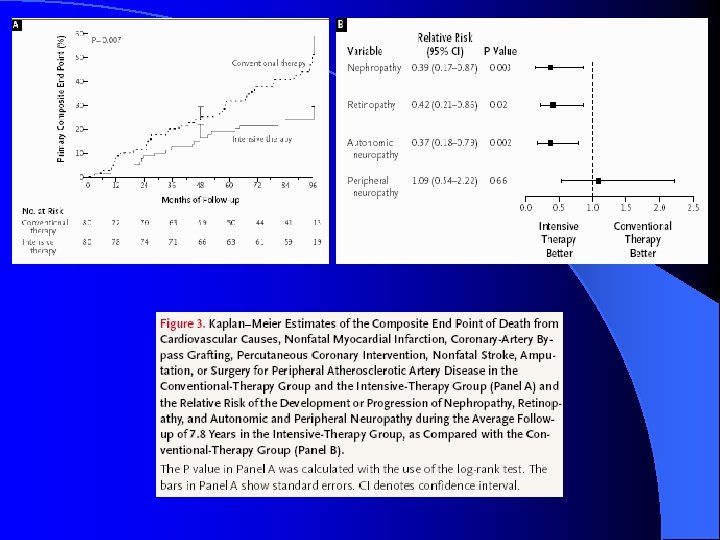
Multifactorial DM Rx: STENO-2 l Jan 2003, NEJM 348: 383 -93 l RCT mimicking real life clinic l 160 T 2 DM patients with microalbuminuria l Randomized: Conventional Rx as per National Guidelines versus l Intensive Rx l • Behaviour modification • Pharmacotherapy: targeting BS, BP, Lipids, proteinuria, ASA (initially 2 prevention only, 1 prevention after 1999)
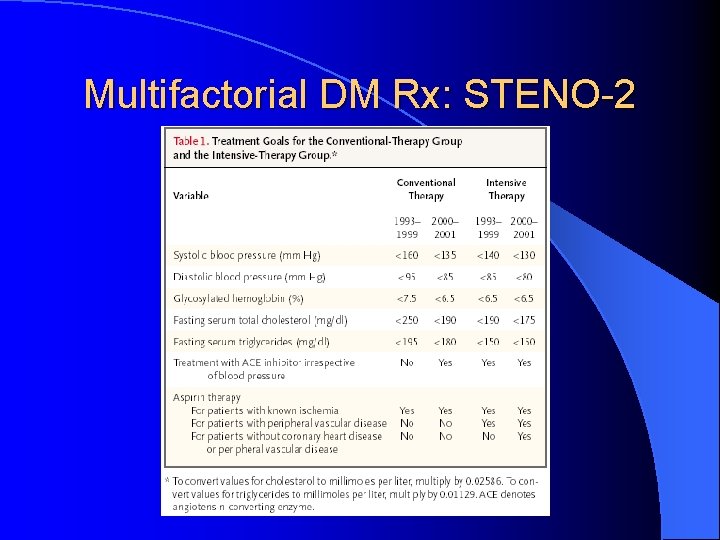
Multifactorial DM Rx: STENO-2
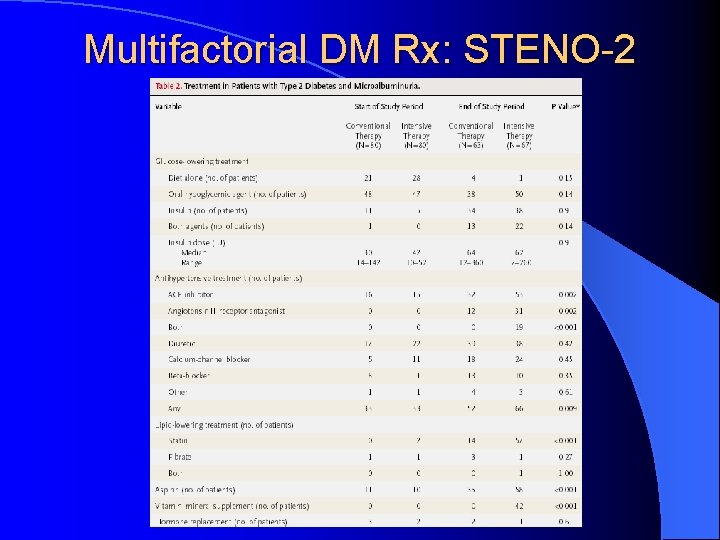
Multifactorial DM Rx: STENO-2
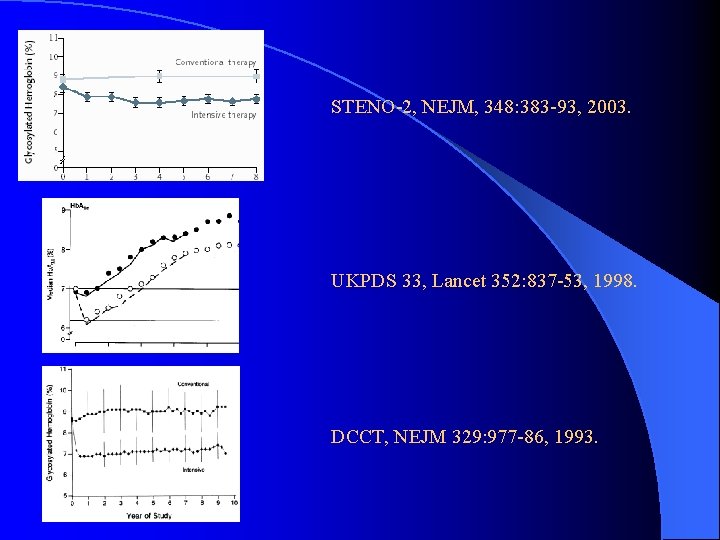
STENO-2, NEJM, 348: 383 -93, 2003. UKPDS 33, Lancet 352: 837 -53, 1998. DCCT, NEJM 329: 977 -86, 1993.
Multifactorial DM Rx: STENO-2

Bottom Line: T 2 DM Surveillance l l l Hb. A 1 c, BP: q 3 mos UMALB/Creat ratio: q 6 mos - q 1 y Dilated eye exam q 1 -2 y Feet exam at least q 1 y Cardiac Gx. T: l as indicated (symptoms, prior to new exercise regime)
Bottom Line: T 2 DM Intervention l BP cntrl: l l Target: < 130/80 (< 120/75 if proteinuria > 1 g/d) 1 st Line: – HOPE criteria met: ACE-I, ARB if ACE-I intolerant > 55 y & 1 CV risk factor: HTN, microalbuminuria, current smoker, TC > 5. 2 m. M, HDL < 0. 9 m. M – No HOPE criteria: ARB l l l 2 nd Line: Thiazide 3 rd Line: -blocker or DHP-CCB (Norvasc, Plendil) Combine ACE-I + ARB ? (watch K+!) – CHF (as per CHARM or Val-He. FT) – Overt nephropathy + HOPE criteria met l Angiotensin II blockade: l Altace even if normal BP if HOPE criteria met
Bottom Line: T 2 DM Intervention l Proteinuria l l l BP < 120/75, Hb. A 1 c < 7. 0 % No HOPE: ARB HOPE Criteria met, microalbuminuria: ACE-I HOPE Criteria met, macroalbuminuria: ACE-I + ARB (watch K+!) Lipids l l l age > 40 or vascular disease, regardless of lipid profile Zocor 40 mg/d target LDL 2. 5 m. M Still hi TG, low HDL: consider gemfibrozil or niacin • TG > 6. 0 -11. 0 m. M: risk of pancreatitis ECASA 81 mg/d, Smoking cessation l 2 prevention CAD: -blocker, ACE-I l
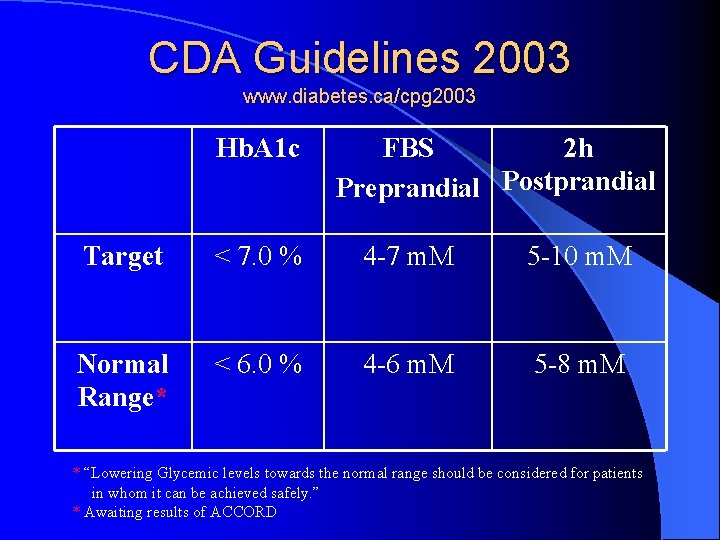
CDA Guidelines 2003 www. diabetes. ca/cpg 2003 Hb. A 1 c FBS 2 h Preprandial Postprandial Target < 7. 0 % 4 -7 m. M 5 -10 m. M Normal Range* < 6. 0 % 4 -6 m. M 5 -8 m. M * “Lowering Glycemic levels towards the normal range should be considered for patients in whom it can be achieved safely. ” * Awaiting results of ACCORD
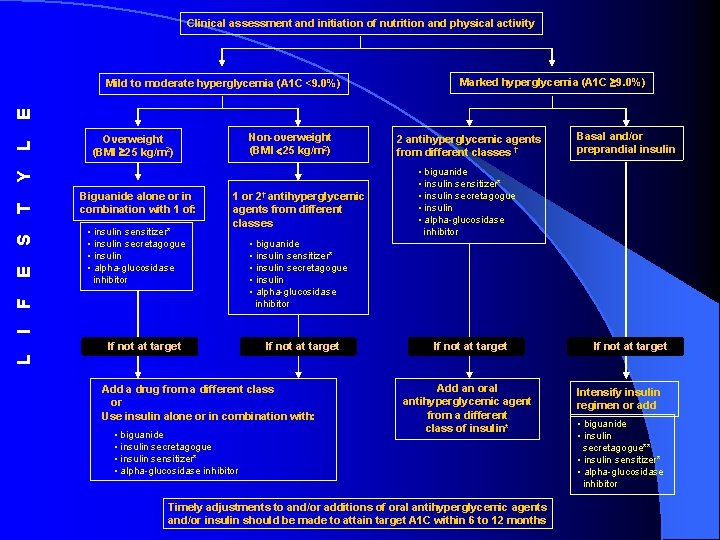
Clinical assessment and initiation of nutrition and physical activity Marked hyperglycemia (A 1 C 9. 0%) Non-overweight (BMI 25 kg/m 2) Overweight (BMI 25 kg/m 2) Biguanide alone or in combination with 1 of: • insulin sensitizer* • insulin secretagogue • insulin • alpha-glucosidase inhibitor 1 or 2† antihyperglycemic agents from different classes 2 antihyperglycemic agents from different classes † Basal and/or preprandial insulin • biguanide • insulin sensitizer* • insulin secretagogue • insulin • alpha-glucosidase inhibitor I F E S T Y L E Mild to moderate hyperglycemia (A 1 C <9. 0%) If not at target L If not at target Add a drug from a different class or Use insulin alone or in combination with: • biguanide • insulin secretagogue • insulin sensitizer* • alpha-glucosidase inhibitor Add an oral antihyperglycemic agent from a different class of insulin* Timely adjustments to and/or additions of oral antihyperglycemic agents and/or insulin should be made to attain target A 1 C within 6 to 12 months Intensify insulin regimen or add • biguanide • insulin secretagogue** • insulin sensitizer* • alpha-glucosidase inhibitor
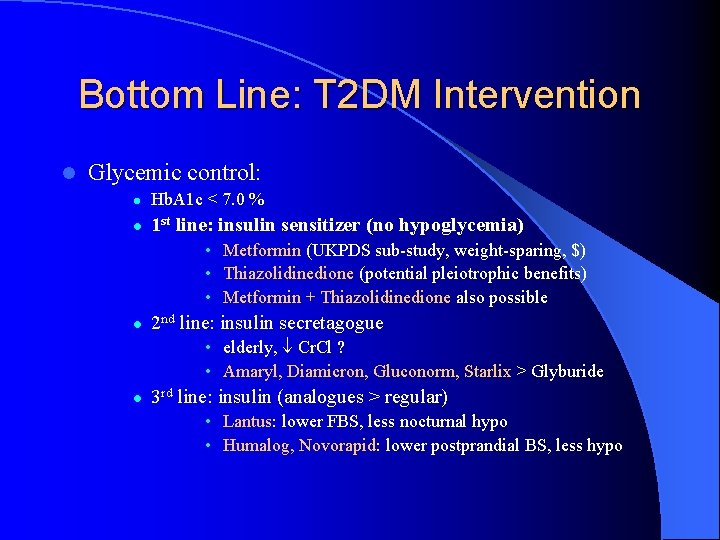
Bottom Line: T 2 DM Intervention l Glycemic control: l Hb. A 1 c < 7. 0 % l 1 st line: insulin sensitizer (no hypoglycemia) • Metformin (UKPDS sub-study, weight-sparing, $) • Thiazolidinedione (potential pleiotrophic benefits) • Metformin + Thiazolidinedione also possible l 2 nd line: insulin secretagogue • elderly, Cr. Cl ? • Amaryl, Diamicron, Gluconorm, Starlix > Glyburide l 3 rd line: insulin (analogues > regular) • Lantus: lower FBS, less nocturnal hypo • Humalog, Novorapid: lower postprandial BS, less hypo

The End
- Slides: 65