Two models formation of secondary organic aerosol Classical

- Slides: 16

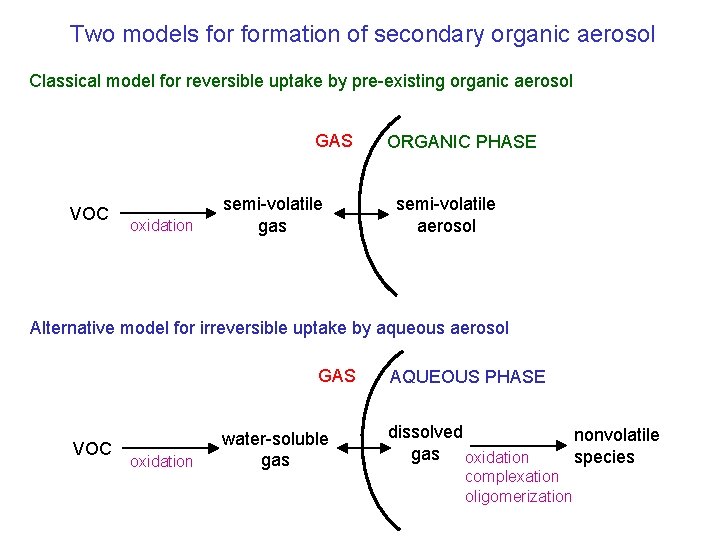
Two models formation of secondary organic aerosol Classical model for reversible uptake by pre-existing organic aerosol GAS VOC oxidation semi-volatile gas ORGANIC PHASE semi-volatile aerosol Alternative model for irreversible uptake by aqueous aerosol GAS VOC oxidation water-soluble gas AQUEOUS PHASE dissolved gas oxidation complexation oligomerization nonvolatile species

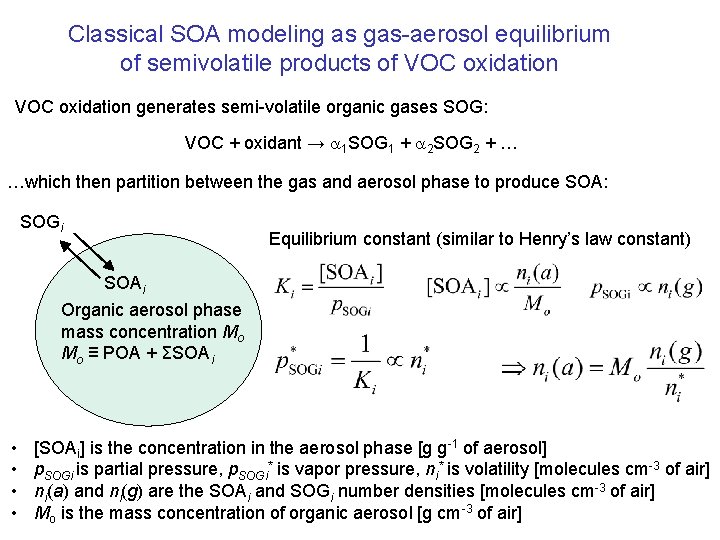
Classical SOA modeling as gas-aerosol equilibrium of semivolatile products of VOC oxidation generates semi-volatile organic gases SOG: VOC + oxidant → 1 SOG 1 + 2 SOG 2 + … …which then partition between the gas and aerosol phase to produce SOA: SOGi Equilibrium constant (similar to Henry’s law constant) SOAi Organic aerosol phase mass concentration Mo Mo ≡ POA + ΣSOAi • • [SOAi] is the concentration in the aerosol phase [g g-1 of aerosol] p. SOGi is partial pressure, p. SOGi* is vapor pressure, ni* is volatility [molecules cm-3 of air] ni(a) and ni(g) are the SOAi and SOGi number densities [molecules cm-3 of air] Mo is the mass concentration of organic aerosol [g cm-3 of air]

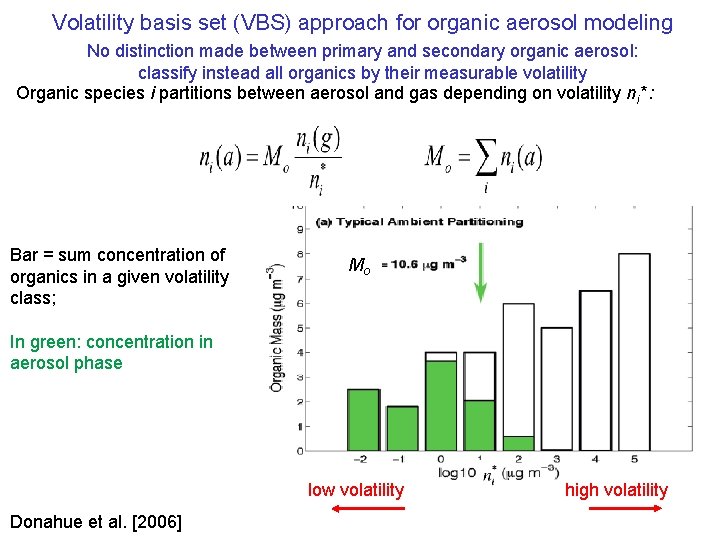
Volatility basis set (VBS) approach for organic aerosol modeling No distinction made between primary and secondary organic aerosol: classify instead all organics by their measurable volatility Organic species i partitions between aerosol and gas depending on volatility ni* : Bar = sum concentration of organics in a given volatility class; Mo In green: concentration in aerosol phase low volatility Donahue et al. [2006] high volatility

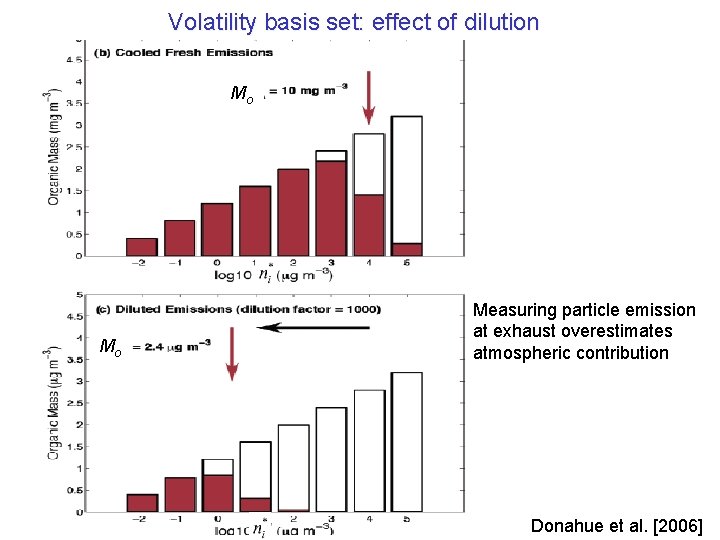
Volatility basis set: effect of dilution Mo Mo Measuring particle emission at exhaust overestimates atmospheric contribution Donahue et al. [2006]

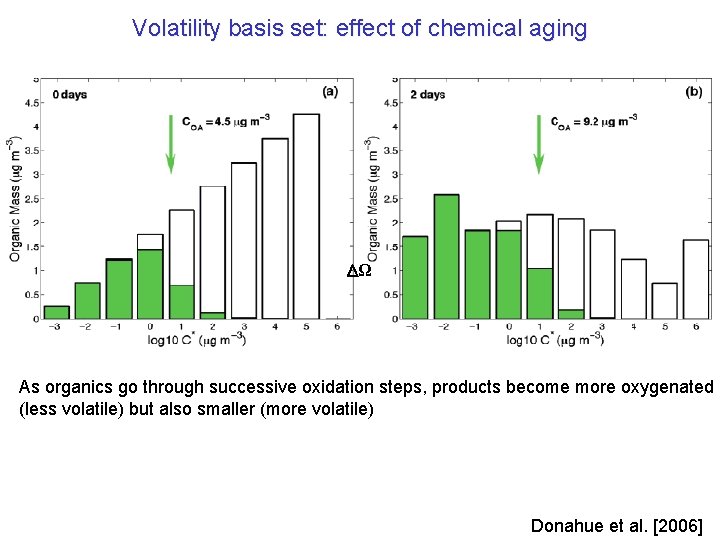
Volatility basis set: effect of chemical aging Ω As organics go through successive oxidation steps, products become more oxygenated (less volatile) but also smaller (more volatile) Donahue et al. [2006]

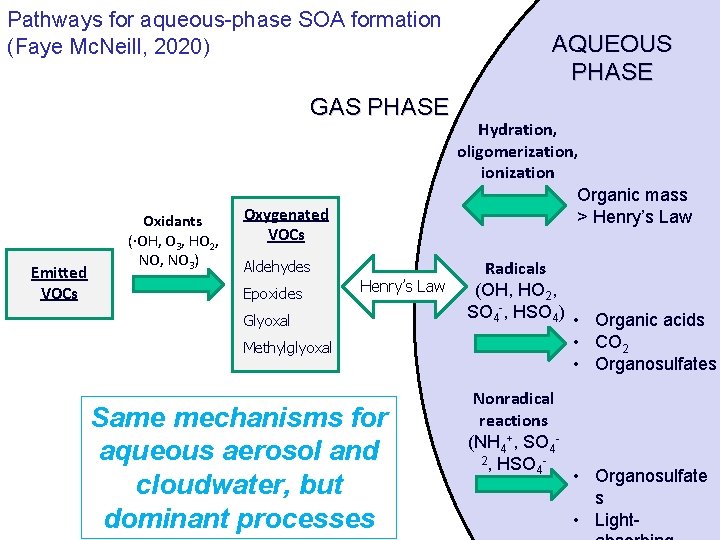
Pathways for aqueous-phase SOA formation (Faye Mc. Neill, 2020) GAS PHASE Emitted VOCs Oxidants (·OH, O 3, HO 2, NO 3) Oxygenated VOCs Aldehydes Epoxides Henry’s Law Glyoxal Methylglyoxal Same mechanisms for aqueous aerosol and cloudwater, but dominant processes AQUEOUS PHASE Hydration, oligomerization, ionization Organic mass > Henry’s Law Radicals (OH, HO 2, SO 4 -, HSO 4) • Organic acids • CO 2 • Organosulfates Nonradical reactions (NH 4+, SO 42, HSO 4 • Organosulfate s • Light-

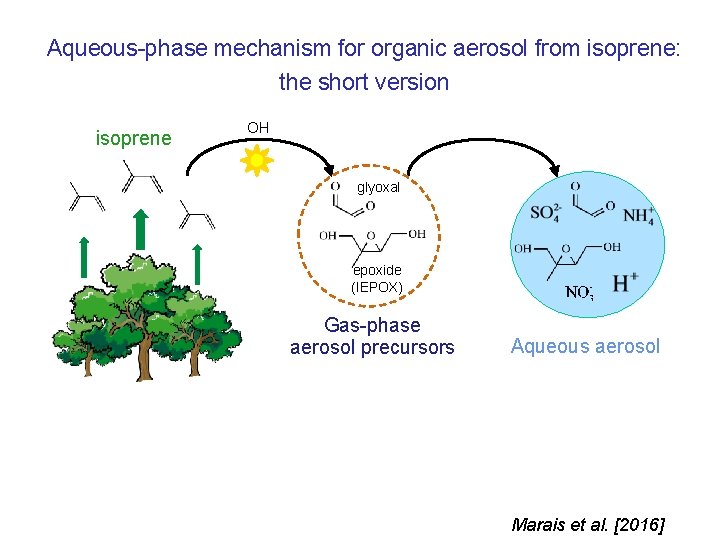
Aqueous-phase mechanism for organic aerosol from isoprene: the short version isoprene OH glyoxal epoxide (IEPOX) Gas-phase aerosol precursors Aqueous aerosol Marais et al. [2016]

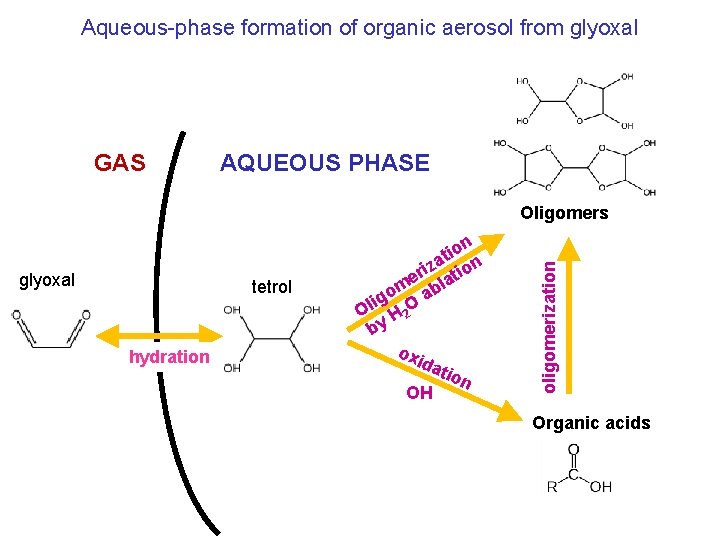
Aqueous-phase formation of organic aerosol from glyoxal GAS AQUEOUS PHASE glyoxal tetrol hydration on i t iza tion r me abla o ig Ol H 2 O by oxi dat ion OH oligomerization Oligomers Organic acids

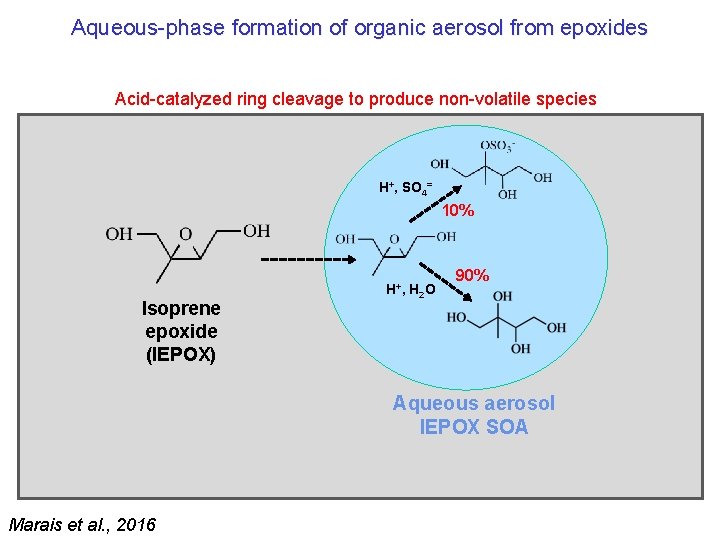
Aqueous-phase formation of organic aerosol from epoxides Acid-catalyzed ring cleavage to produce non-volatile species H+, SO 4= 10% Isoprene epoxide (IEPOX) H+, H 2 O 90% Aqueous aerosol IEPOX SOA Marais et al. , 2016

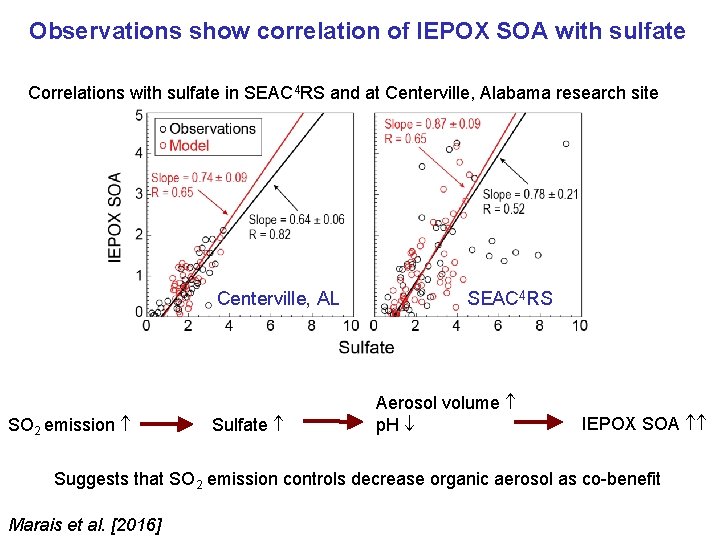
Observations show correlation of IEPOX SOA with sulfate Correlations with sulfate in SEAC 4 RS and at Centerville, Alabama research site Centerville, AL SO 2 emission Sulfate SEAC 4 RS Aerosol volume p. H IEPOX SOA Suggests that SO 2 emission controls decrease organic aerosol as co-benefit Marais et al. [2016]

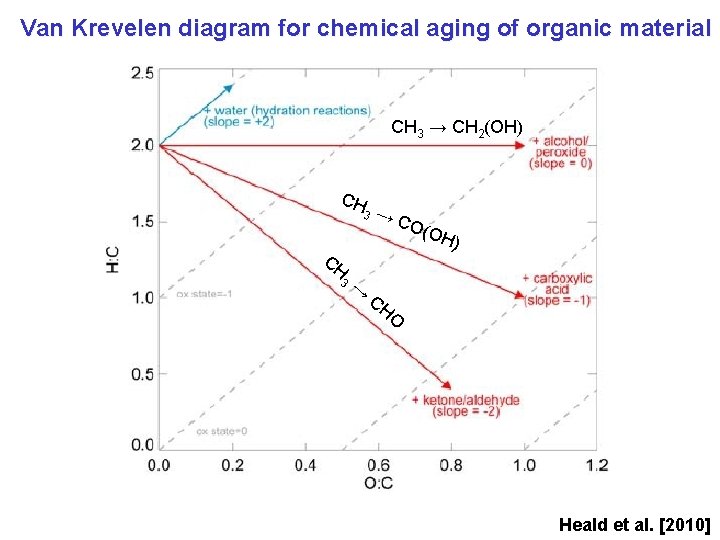
Van Krevelen diagram for chemical aging of organic material CH 3 → CH 2(OH) CH 3 → →C O(O H) CH O Heald et al. [2010]

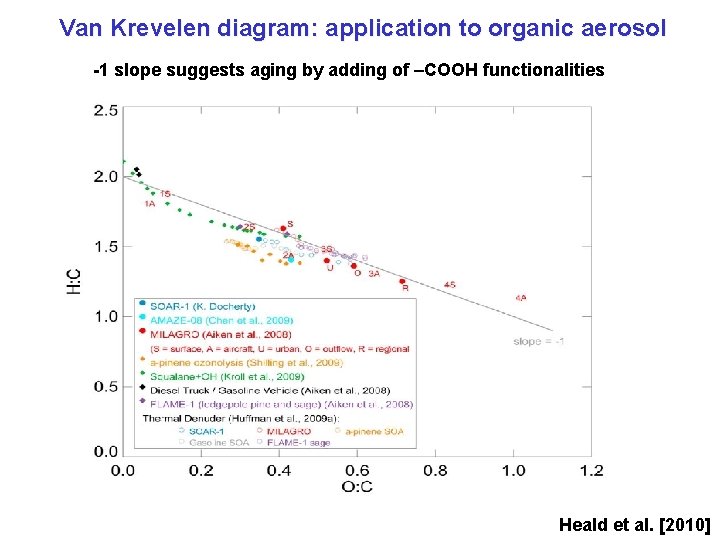
Van Krevelen diagram: application to organic aerosol -1 slope suggests aging by adding of –COOH functionalities Heald et al. [2010]

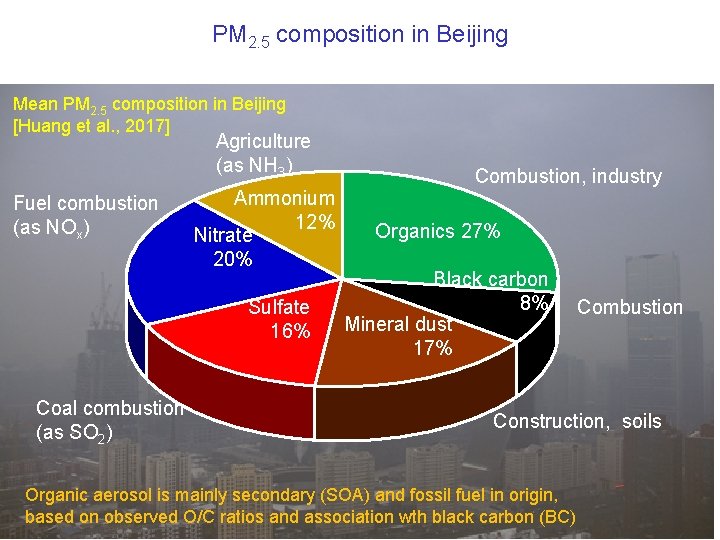
PM 2. 5 composition in Beijing Mean PM 2. 5 composition in Beijing [Huang et al. , 2017] Agriculture (as NH 3) Fuel combustion (as NOx) Ammonium 12% Nitrate 20% Sulfate 16% Coal combustion (as SO 2) Combustion, industry Organics 27% Black carbon 8% Mineral dust 17% Combustion Construction, soils Organic aerosol is mainly secondary (SOA) and fossil fuel in origin, based on observed O/C ratios and association wth black carbon (BC)

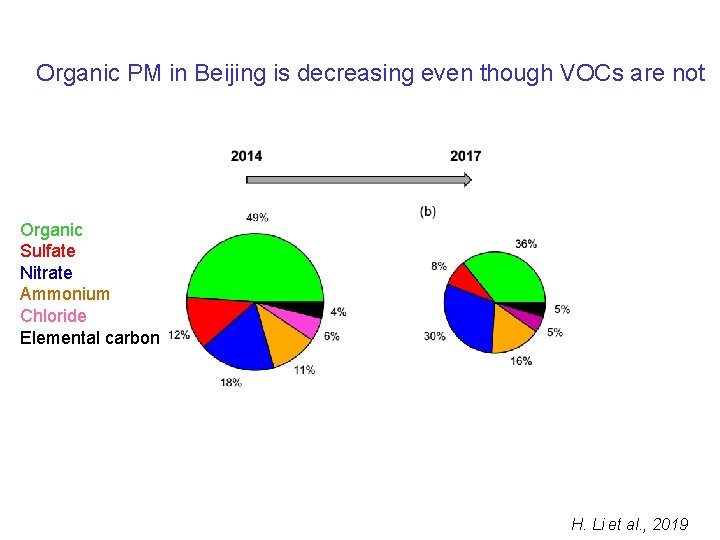
Organic PM in Beijing is decreasing even though VOCs are not Organic Sulfate Nitrate Ammonium Chloride Elemental carbon H. Li et al. , 2019

Beijing winter haze event, 16 -22 December 2016: SOA originates from aqueous-phase oxidation of fossil fuel-derived primary organic aerosol (FFPOA) rather than from oxidation of VOCs moderate RH high RH cold front Highly oxidized aqueous SOA (aq-SOA) is produced rapidly at high RH in correlation with sulfate while fossil fuel POA declines Wang et al. , submitted to PNAS

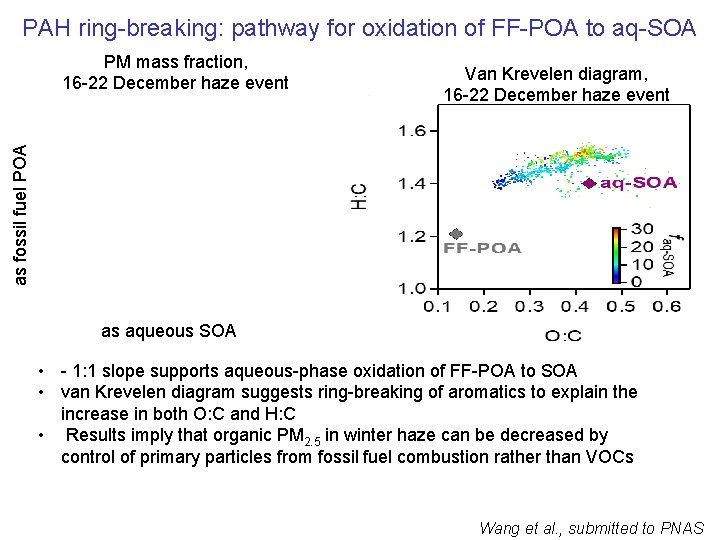
PAH ring-breaking: pathway for oxidation of FF-POA to aq-SOA Van Krevelen diagram, 16 -22 December haze event as fossil fuel POA PM mass fraction, 16 -22 December haze event as aqueous SOA • - 1: 1 slope supports aqueous-phase oxidation of FF-POA to SOA • van Krevelen diagram suggests ring-breaking of aromatics to explain the increase in both O: C and H: C • Results imply that organic PM 2. 5 in winter haze can be decreased by control of primary particles from fossil fuel combustion rather than VOCs Wang et al. , submitted to PNAS