Two different heats measured in heatingcooling curves for


- Slides: 20

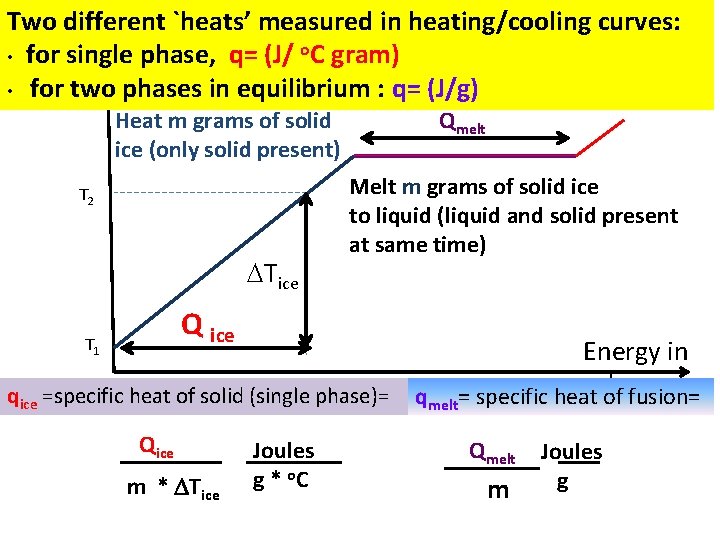
Two different `heats’ measured in heating/cooling curves: • for single phase, q= (J/ o. C gram) • for two phases in equilibrium : q= (J/g) Heat m grams of solid ice (only solid present) T 2 Tice Qmelt Melt m grams of solid ice to liquid (liquid and solid present at same time) Q ice T 1 qice =specific heat of solid (single phase)= Qice m * Tice Joules g * o. C qmelt Energy in calof fusion= = specific heat Qmelt m Joules g

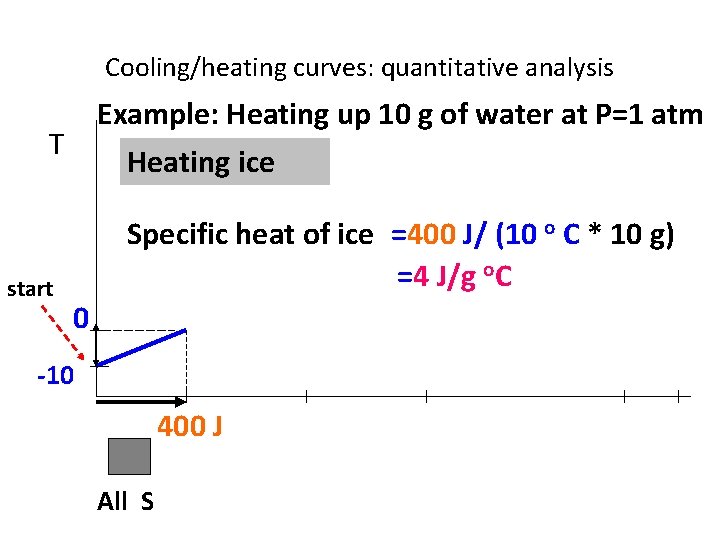
Cooling/heating curves: quantitative analysis Example: Heating up 10 g of water at P=1 atm T start Heating ice Specific heat of ice =400 J/ (10 o C * 10 g) =4 J/g o. C 0 -10 400 J All S

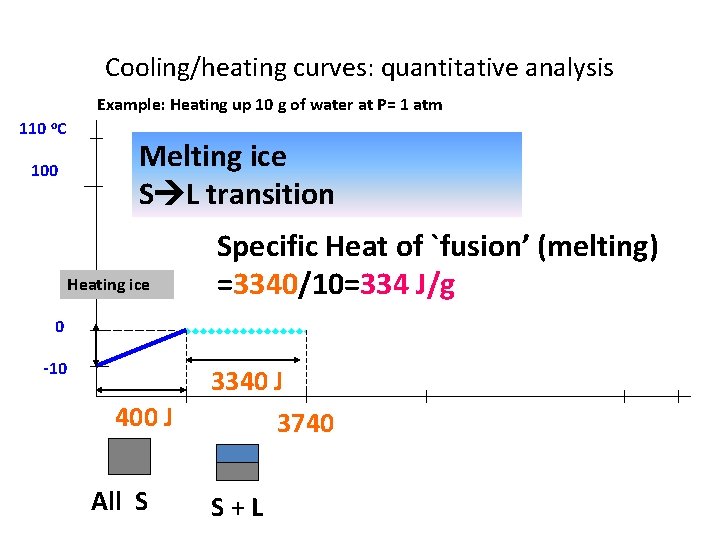
Cooling/heating curves: quantitative analysis Example: Heating up 10 g of water at P= 1 atm 110 o. C 100 Melting ice S L transition Heating ice Specific Heat of `fusion’ (melting) =3340/10=334 J/g 0 -10 400 J All S 3340 J 3740 S+L

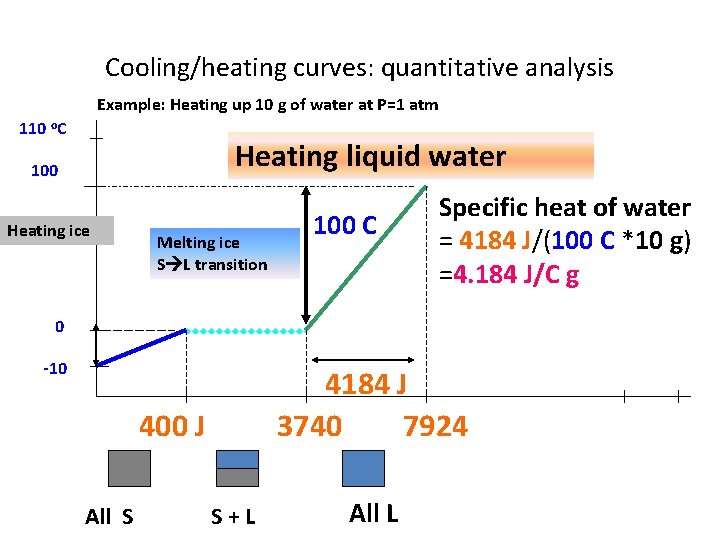
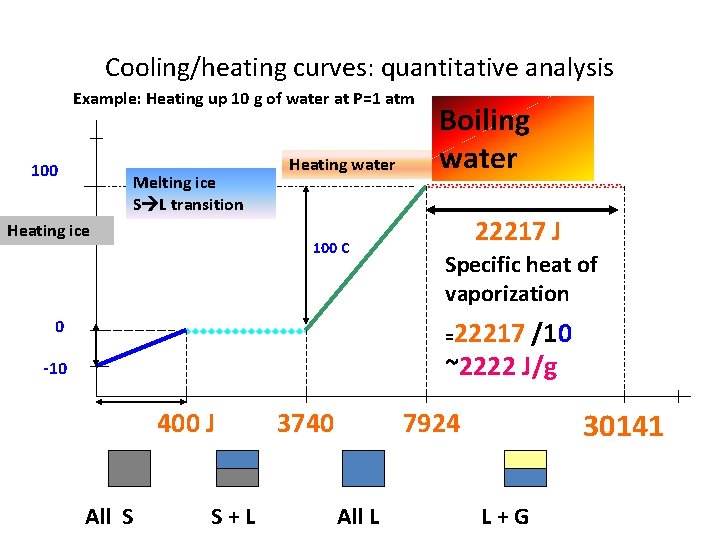
Cooling/heating curves: quantitative analysis Example: Heating up 10 g of water at P=1 atm 110 o. C Heating liquid water 100 Heating ice Melting ice S L transition 100 C Specific heat of water = 4184 J/(100 C *10 g) =4. 184 J/C g 0 -10 4184 J 3740 7924 400 J All S S+L All L

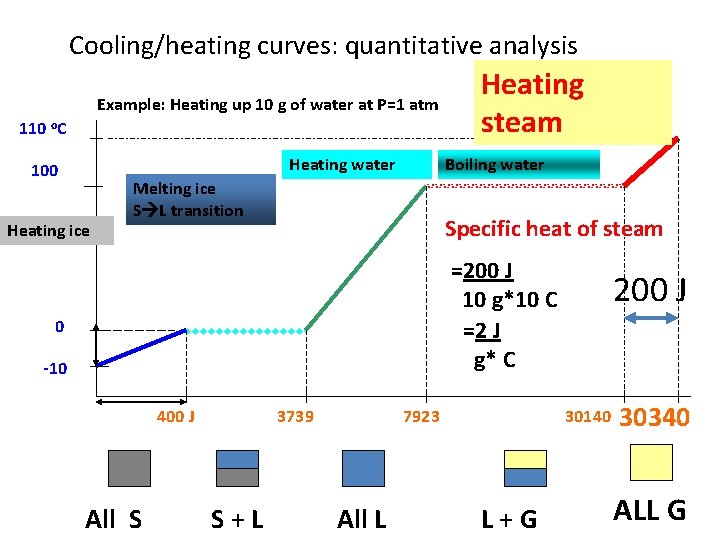
Cooling/heating curves: quantitative analysis Example: Heating up 10 g of water at P=1 atm 100 Melting ice S L transition Heating ice Heating water 100 C Boiling water 22217 J Specific heat of vaporization =22217 /10 ~2222 J/g 0 -10 400 J All S S+L 3740 30141 7924 All L L+G

Cooling/heating curves: quantitative analysis Example: Heating up 10 g of water at P=1 atm 110 o. C Heating water 100 Heating ice Heating steam Boiling water Melting ice S L transition Specific heat of steam =200 J 10 g*10 C =2 J g* C 0 -10 400 J All S 3739 S+L 7923 All L 200 J 30140 L+G 30340 ALL G

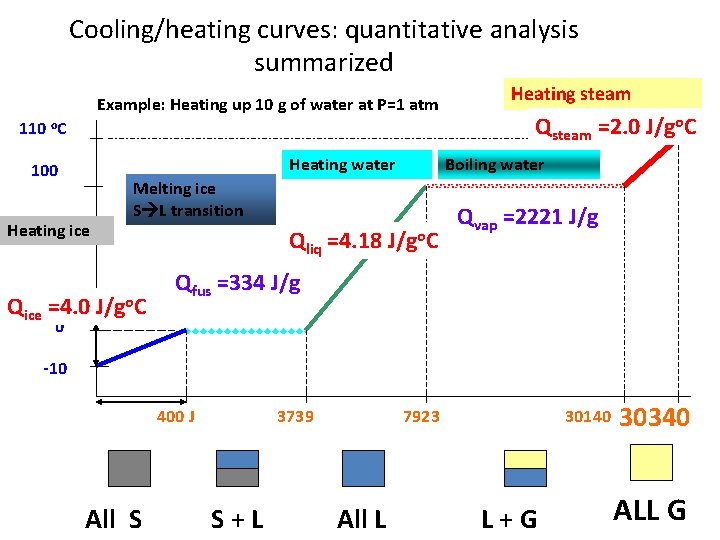
Cooling/heating curves: quantitative analysis summarized Example: Heating up 10 g of water at P=1 atm 110 o. C Heating water 100 Heating ice Qsteam =2. 0 J/go. C Boiling water Melting ice S L transition Qice =4. 0 J/go. C Heating steam Qliq =4. 18 J/go. C Qvap =2221 J/g Qfus =334 J/g 0 -10 400 J All S 3739 S+L 7923 All L 30140 L+G 30340 ALL G

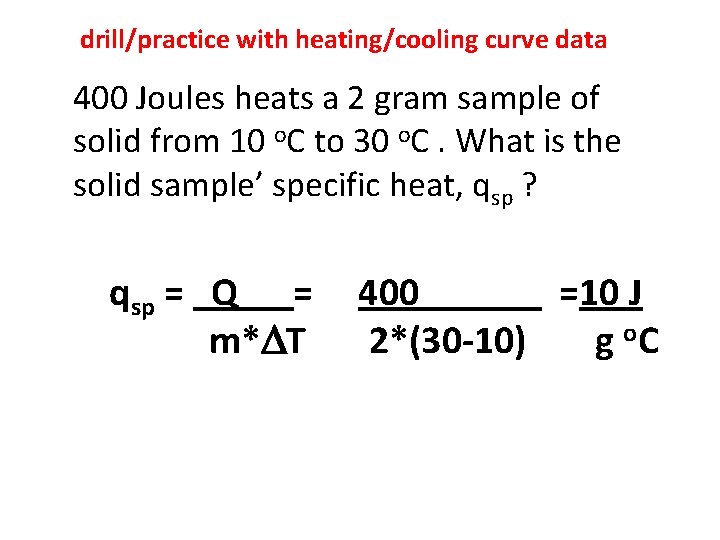
drill/practice with heating/cooling curve data 400 Joules heats a 2 gram sample of solid from 10 o. C to 30 o. C. What is the solid sample’ specific heat, qsp ? qsp = Q = m* T 400 =10 J 2*(30 -10) g o. C

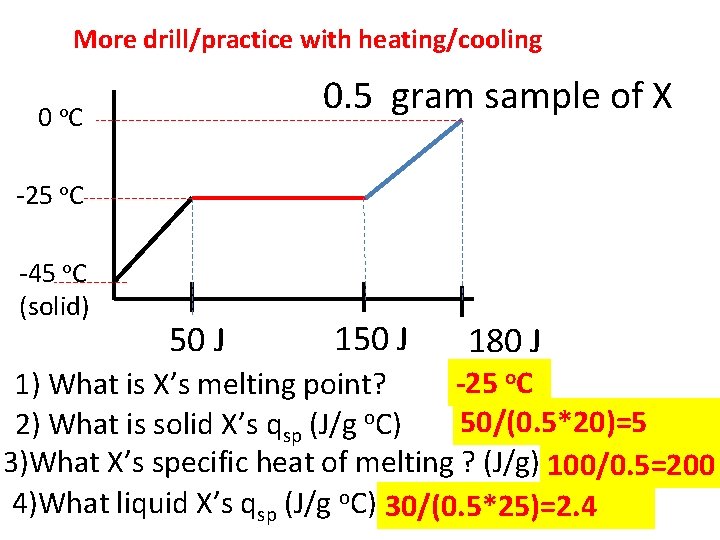
More drill/practice with heating/cooling 0 0. 5 gram sample of X o. C -25 o. C -45 o. C (solid) 50 J 180 J -25 o. C 1) What is X’s melting point? 50/(0. 5*20)=5 2) What is solid X’s qsp (J/g o. C) 3)What X’s specific heat of melting ? (J/g) 100/0. 5=200 4)What liquid X’s qsp (J/g o. C) 30/(0. 5*25)=2. 4

Photo of STS 103 (Space Shuttle) Artist’s conception of heating re-entry Space shuttle tile – An example of very, very high qsp (specific heat capacity) material

SHUTTLE TILE IN MORE DETAIL

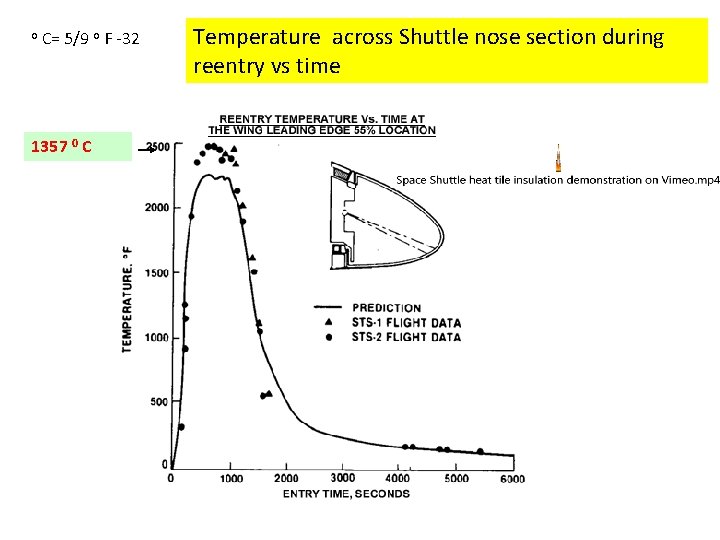
o C= 5/9 o F -32 1357 0 C Temperature across Shuttle nose section during reentry vs time

Three Common Methods of Exploring Phases recounted: 1)P-T vaporization curves (Clausius-Clapeyron eq. ) 2)Heating/cooling curves 3)Phase Diagrams

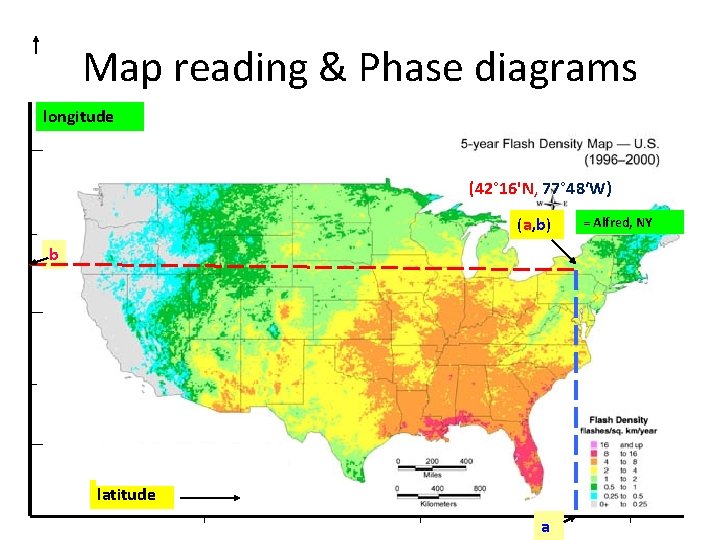
Map reading & Phase diagrams longitude (42° 16'N, 77° 48‘W) (a, b) b latitude a = Alfred, NY

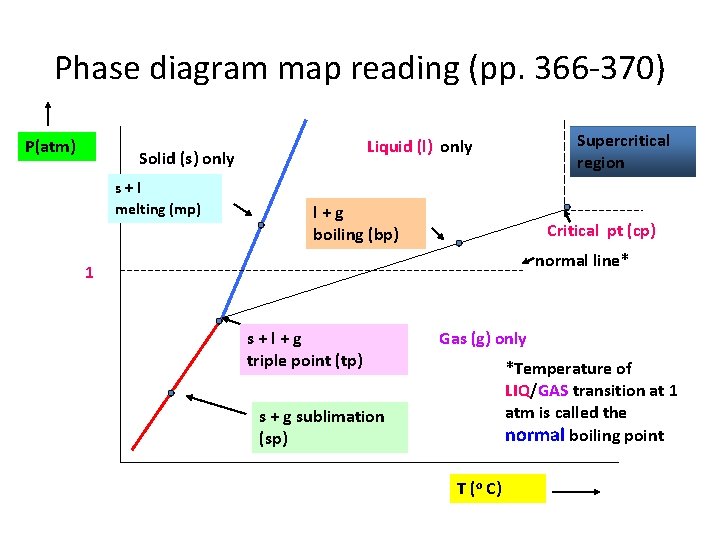
Phase diagram map reading (pp. 366 -370) P(atm) Solid (s) only s+l melting (mp) Supercritical region Liquid (l) only l+g boiling (bp) Critical pt (cp) normal line* 1 s+l+g triple point (tp) Gas (g) only *Temperature of LIQ/GAS transition at 1 atm is called the normal boiling point s + g sublimation (sp) T (o C)

Pressure is often under appreciated as a factor in phase changes Cryophorous demo dramatic visual of phase changes as T, P change

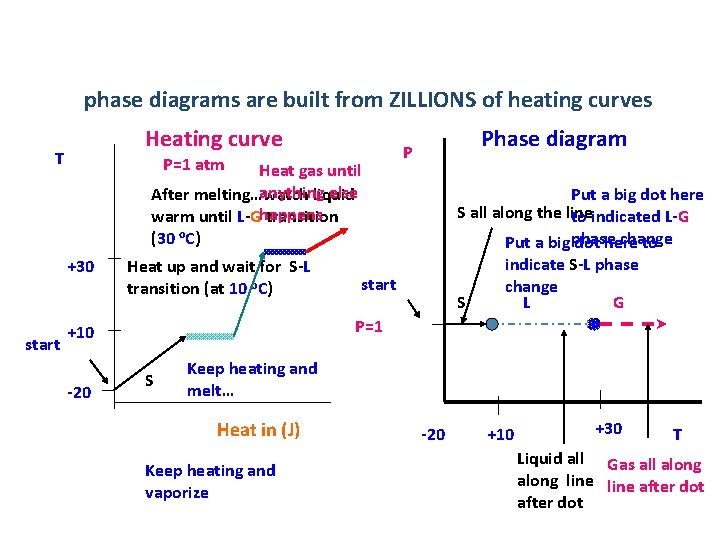
phase diagrams are built from ZILLIONS of heating curves Heating curve T P=1 atm Heat gas until anything else After melting…watch liquid warm until L-Ghappens transition (30 o. C) +30 start Heat up and wait for S-L transition (at 10 o. C) Put a big dot here S all along the line to indicated L-G change Put a big phase dot here to indicate S-L phase change S L G start P=1 +10 -20 Phase diagram P S Keep heating and melt… Heat in (J) Keep heating and vaporize -20 +10 +30 T Liquid all Gas all along line after dot

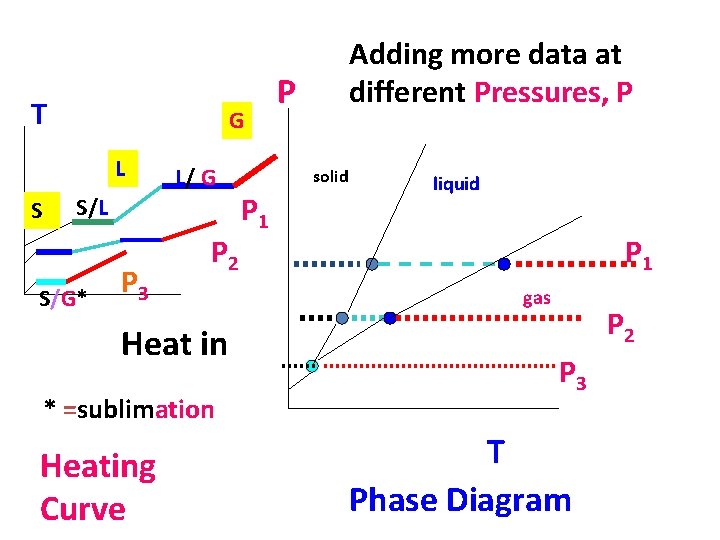
T G L S L/ G S/L S/G* P 3 P 2 Heat in * =sublimation Heating Curve Adding more data at different Pressures, P P solid P 1 liquid P 1 gas P 2 P 3 T Phase Diagram

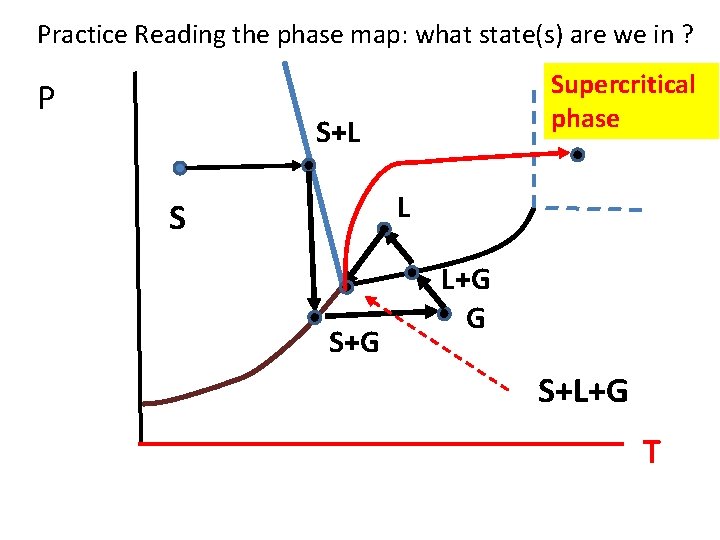
Practice Reading the phase map: what state(s) are we in ? P Supercritical phase S+L L S S+G L+G G S+L+G T

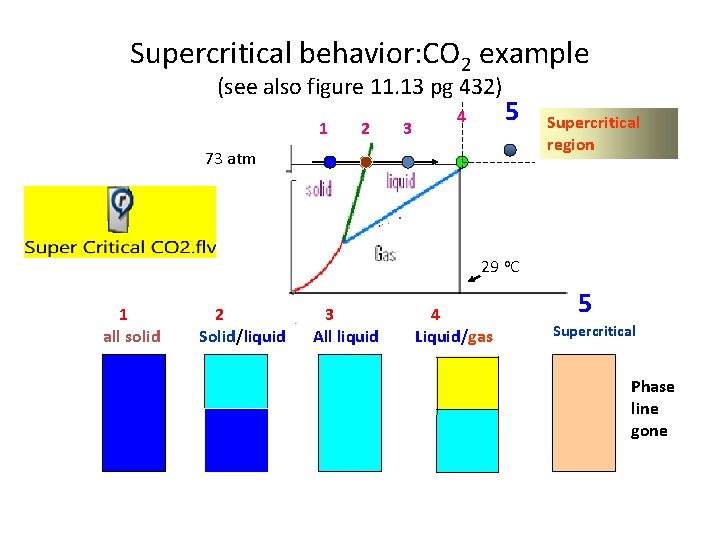
Supercritical behavior: CO 2 example (see also figure 11. 13 pg 432) 1 2 3 4 5 73 atm Supercritical region 29 o. C 1 all solid 2 Solid/liquid 3 All liquid 4 Liquid/gas 5 Supercritical Phase line gone