Tumor immunotherapies To stimulate and enhance tumorspecific immunity

















- Slides: 60

Tumor immunotherapies: To stimulate and enhance tumor-specific immunity

T U M O R T u m o r ( n e o p l a s i a ) :

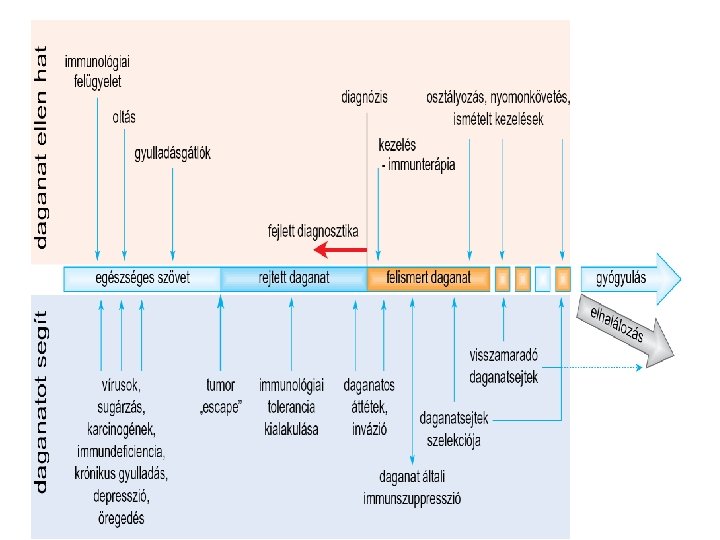
Tumors and the immune system How does the immune system eliminate cancer cells? How do cancer cells escape from Immunosurveilance? How can we help to win the battle between immune system and cancers?

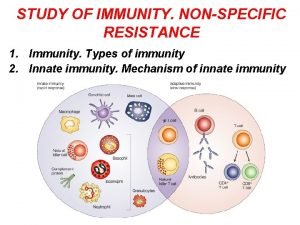
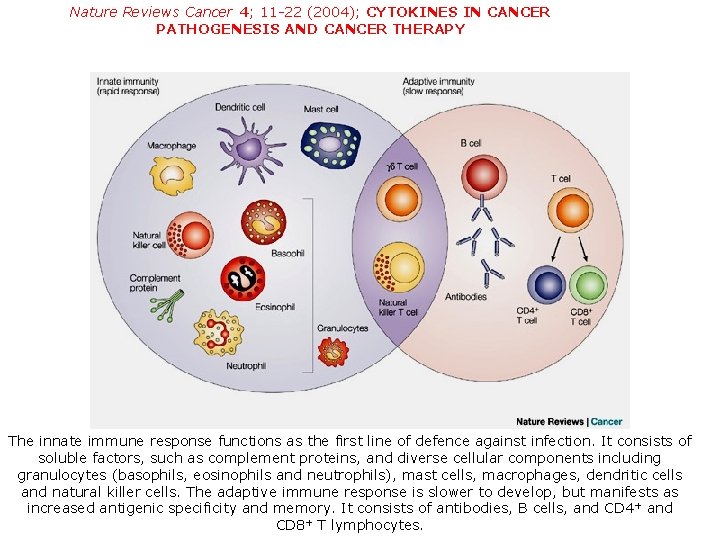
Nature Reviews Cancer 4; 11 -22 (2004); CYTOKINES IN CANCER PATHOGENESIS AND CANCER THERAPY The innate immune response functions as the first line of defence against infection. It consists of soluble factors, such as complement proteins, and diverse cellular components including granulocytes (basophils, eosinophils and neutrophils), mast cells, macrophages, dendritic cells and natural killer cells. The adaptive immune response is slower to develop, but manifests as increased antigenic specificity and memory. It consists of antibodies, B cells, and CD 4 + and CD 8+ T lymphocytes.

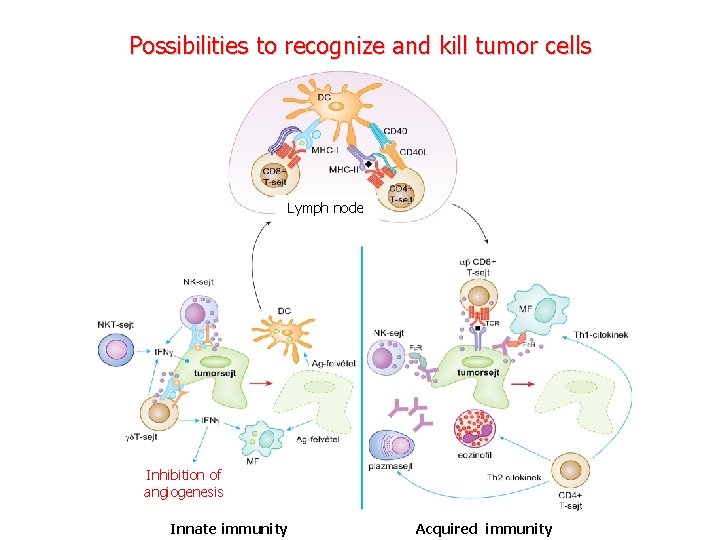
Possibilities to recognize and kill tumor cells Lymph node Inhibition of angiogenesis Innate immunity Acquired immunity

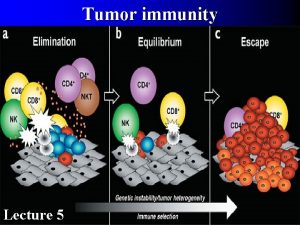
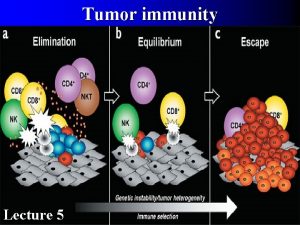
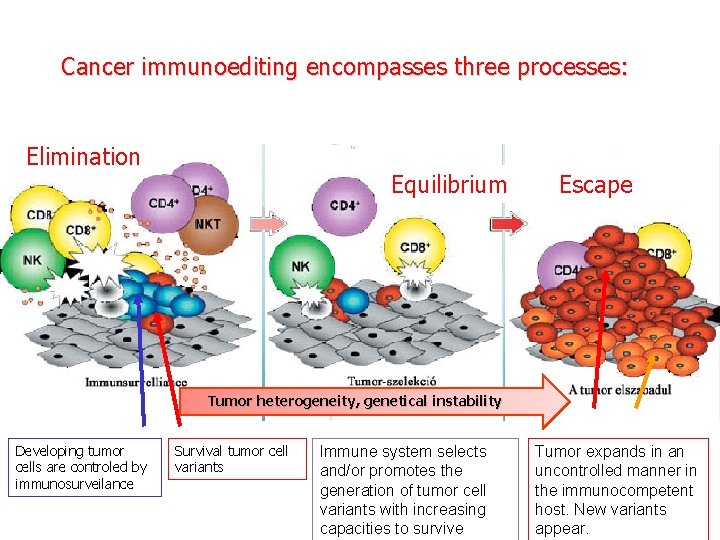
Cancer immunoediting encompasses three processes: Elimination Equilibrium Escape Tumor heterogeneity, genetical instability Developing tumor cells are controled by immunosurveilance Survival tumor cell variants Immune system selects and/or promotes the generation of tumor cell variants with increasing capacities to survive Tumor expands in an uncontrolled manner in the immunocompetent host. New variants appear.

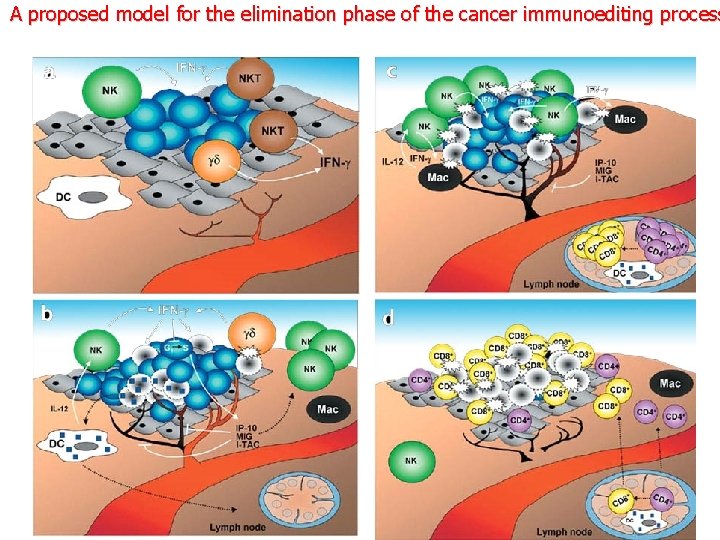
A proposed model for the elimination phase of the cancer immunoediting process

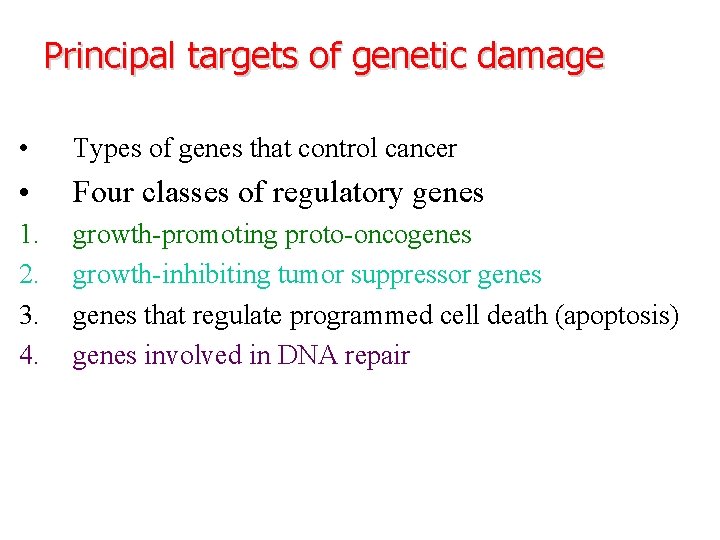
Principal targets of genetic damage • Types of genes that control cancer • Four classes of regulatory genes 1. 2. 3. 4. growth-promoting proto-oncogenes growth-inhibiting tumor suppressor genes that regulate programmed cell death (apoptosis) genes involved in DNA repair

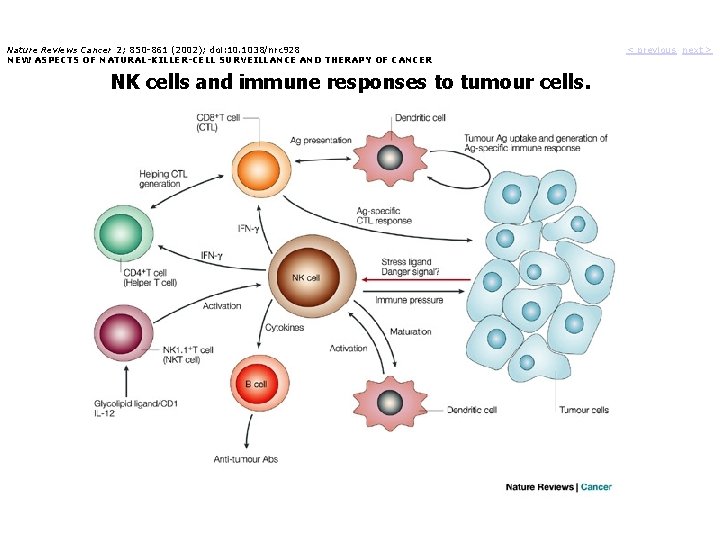
Nature Reviews Cancer 2; 850 -861 (2002); doi: 10. 1038/nrc 928 NEW ASPECTS OF NATURAL-KILLER-CELL SURVEILLANCE AND THERAPY OF CANCER NK cells and immune responses to tumour cells. < previous next >

- Many tumors do elicit an immune antigens response due to tumor - Many tumors evade host immune response through several mechanisms

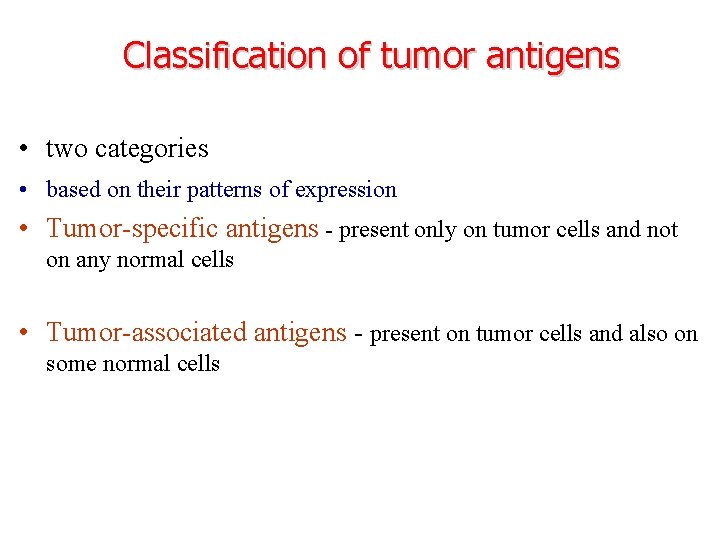
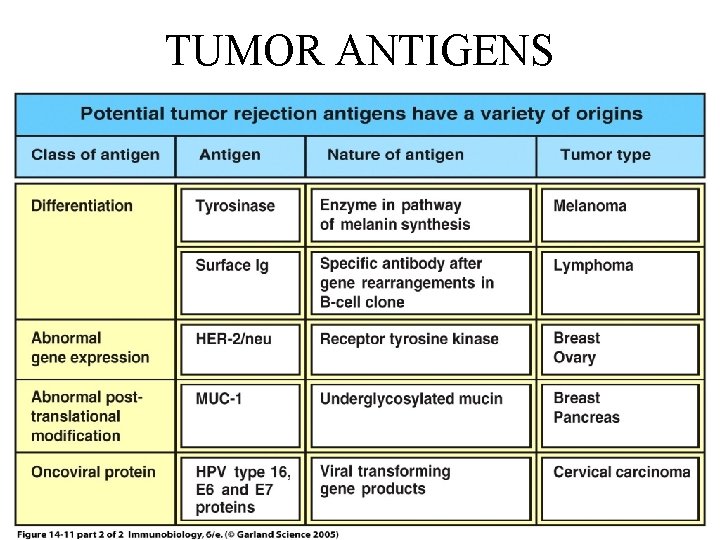
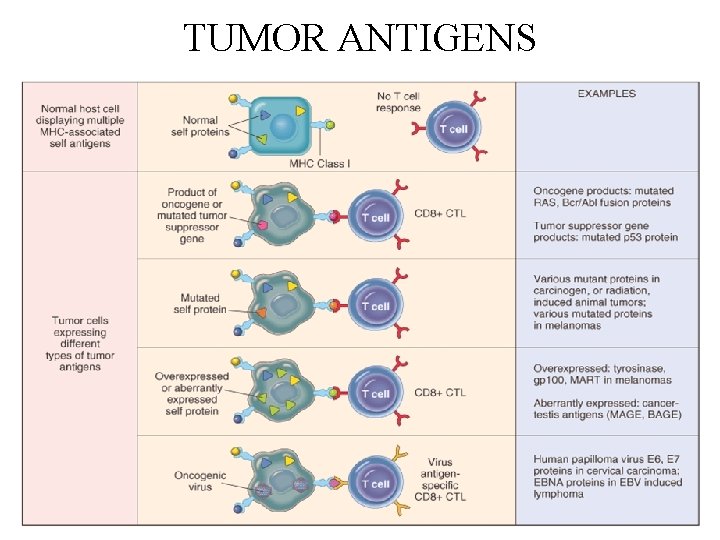
Classification of tumor antigens • two categories • based on their patterns of expression • Tumor-specific antigens - present only on tumor cells and not on any normal cells • Tumor-associated antigens - present on tumor cells and also on some normal cells

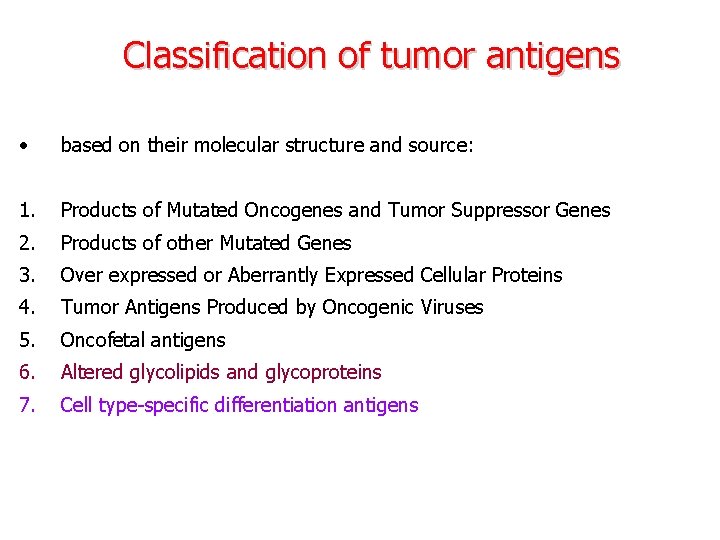
Classification of tumor antigens • based on their molecular structure and source: 1. Products of Mutated Oncogenes and Tumor Suppressor Genes 2. Products of other Mutated Genes 3. Over expressed or Aberrantly Expressed Cellular Proteins 4. Tumor Antigens Produced by Oncogenic Viruses 5. Oncofetal antigens 6. Altered glycolipids and glycoproteins 7. Cell type-specific differentiation antigens

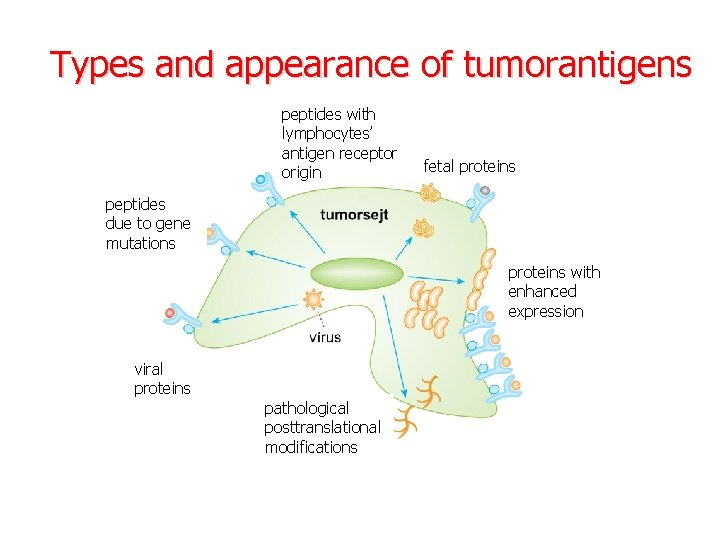
Types and appearance of tumorantigens peptides with lymphocytes’ antigen receptor origin fetal proteins peptides due to gene mutations proteins with enhanced expression viral Viral proteins pathological posttranslational modifications

TUMOR ANTIGENS

TUMOR ANTIGENS



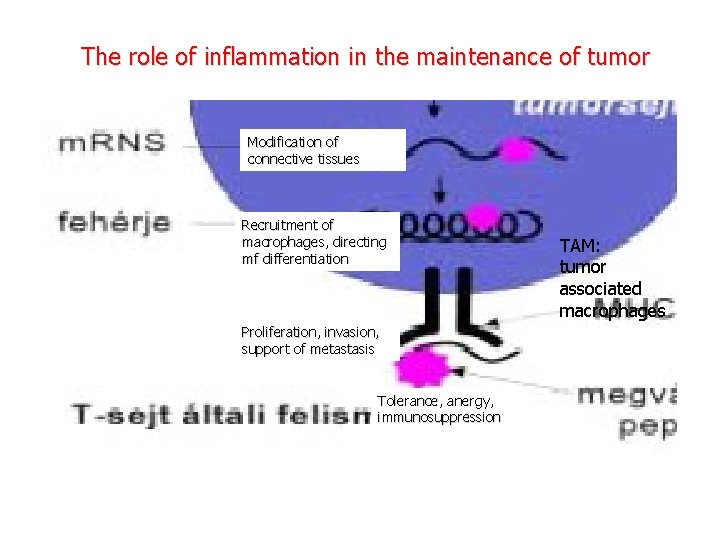
The role of inflammation in the maintenance of tumor Modification of connective tissues Recruitment of macrophages, directing mf differentiation Proliferation, invasion, support of metastasis Tolerance, anergy, immunosuppression TAM: tumor associated macrophages

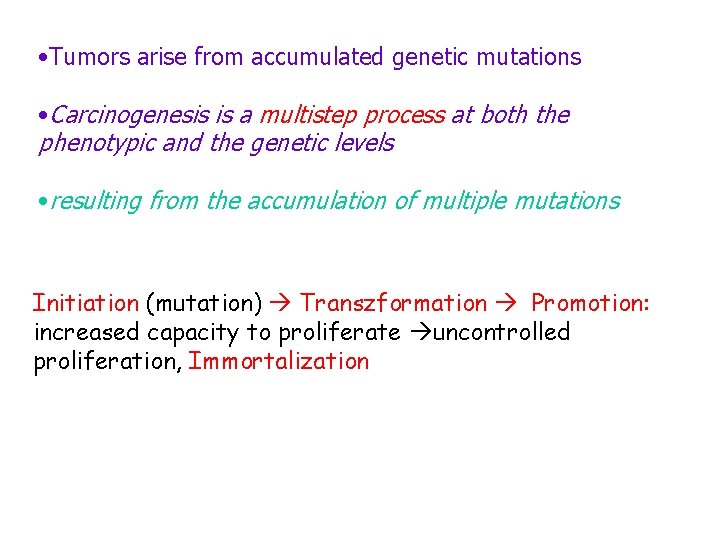
• Tumors arise from accumulated genetic mutations • Carcinogenesis is a multistep process at both the phenotypic and the genetic levels • resulting from the accumulation of multiple mutations Initiation (mutation) Transzformation Promotion: increased capacity to proliferate uncontrolled proliferation, Immortalization

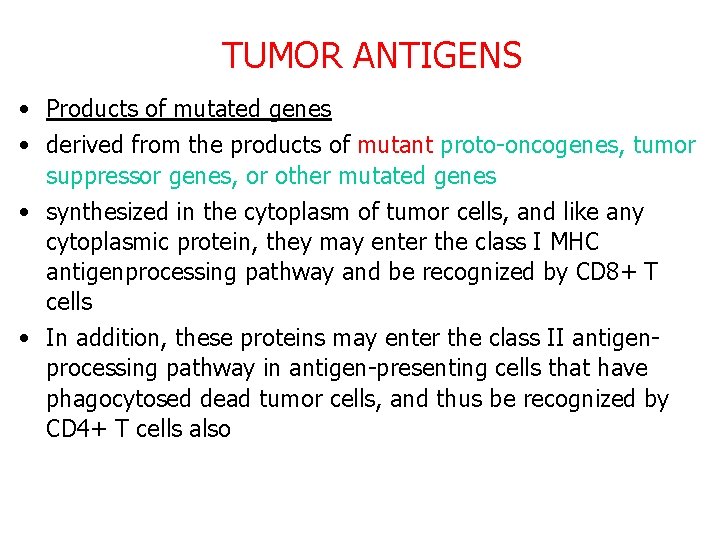
TUMOR ANTIGENS • Products of mutated genes • derived from the products of mutant proto-oncogenes, tumor suppressor genes, or other mutated genes • synthesized in the cytoplasm of tumor cells, and like any cytoplasmic protein, they may enter the class I MHC antigenprocessing pathway and be recognized by CD 8+ T cells • In addition, these proteins may enter the class II antigenprocessing pathway in antigen-presenting cells that have phagocytosed dead tumor cells, and thus be recognized by CD 4+ T cells also

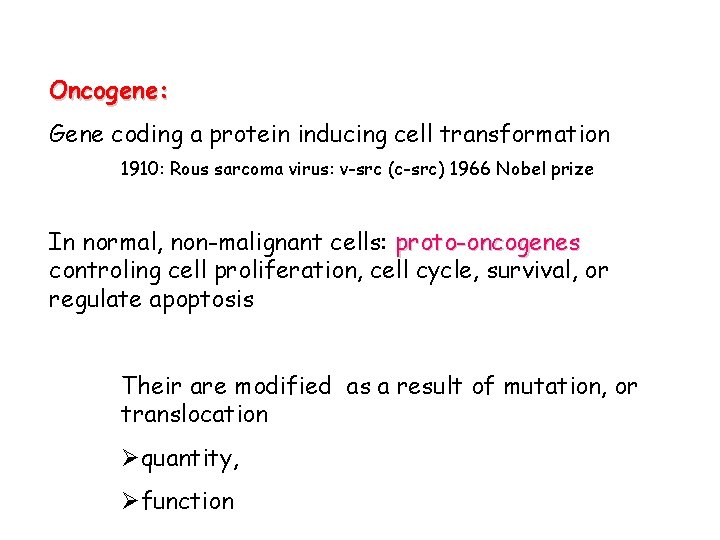
Oncogene: Gene coding a protein inducing cell transformation 1910: Rous sarcoma virus: v-src (c-src) 1966 Nobel prize In normal, non-malignant cells: proto-oncogenes controling cell proliferation, cell cycle, survival, or regulate apoptosis Their are modified as a result of mutation, or translocation Øquantity, Øfunction

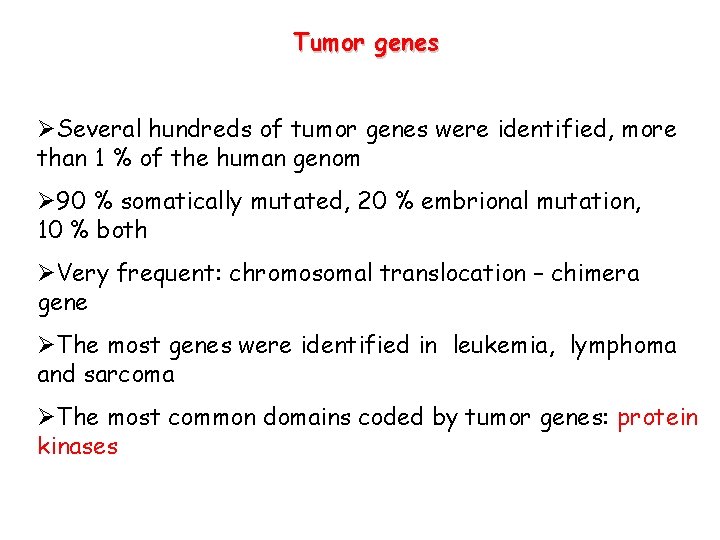
Tumor genes ØSeveral hundreds of tumor genes were identified, more than 1 % of the human genom Ø 90 % somatically mutated, 20 % embrional mutation, 10 % both ØVery frequent: chromosomal translocation – chimera gene ØThe most genes were identified in leukemia, lymphoma and sarcoma ØThe most common domains coded by tumor genes: protein kinases

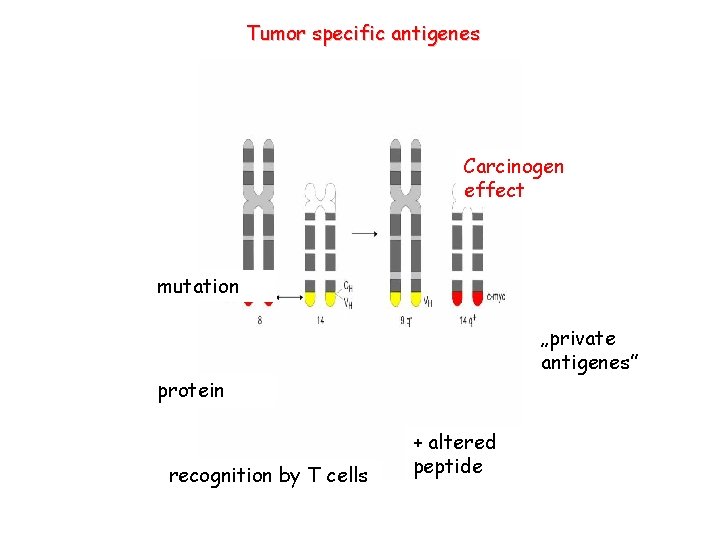
Tumor specific antigenes Carcinogen effect mutation „private antigenes” protein recognition by T cells + altered peptide

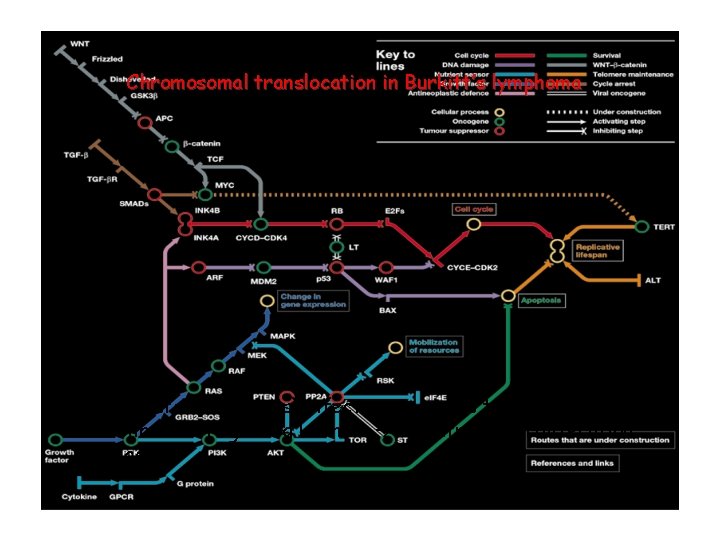
Chromosomal translocation in Burkitt’s lymphoma EBV (Epstein-Bar virus) infection: c-myc is translocated to the enhancer region Ig H chain uncontrolled proliferation of B cells

Specific molecular pathways (subway lines) are responsible for programming malignant transformation behaviours

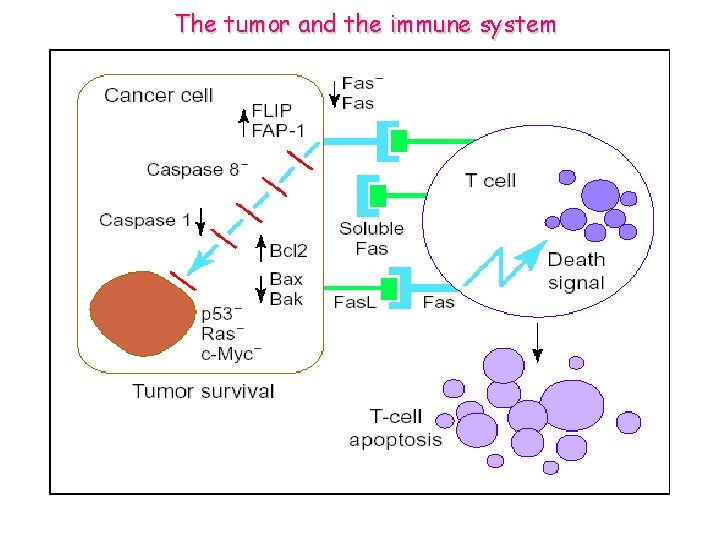
The tumor and the immune system

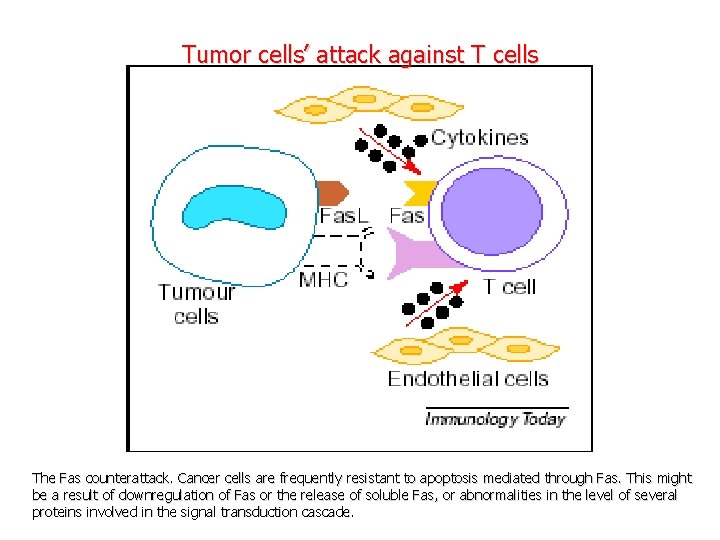
Tumor cells’ attack against T cells The Fas counterattack. Cancer cells are frequently resistant to apoptosis mediated through Fas. This might be a result of downregulation of Fas or the release of soluble Fas, or abnormalities in the level of several proteins involved in the signal transduction cascade.

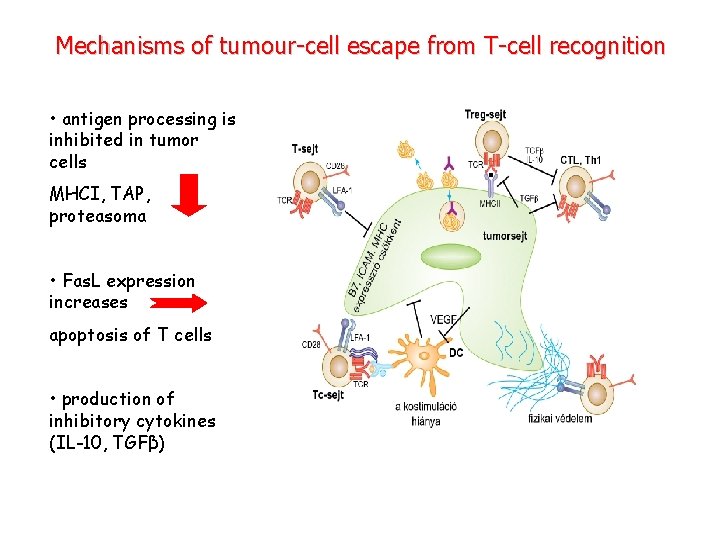
Mechanisms of tumour-cell escape from T-cell recognition • antigen processing is inhibited in tumor cells MHCI, TAP, proteasoma • Fas. L expression increases apoptosis of T cells • production of inhibitory cytokines (IL-10, TGFβ)

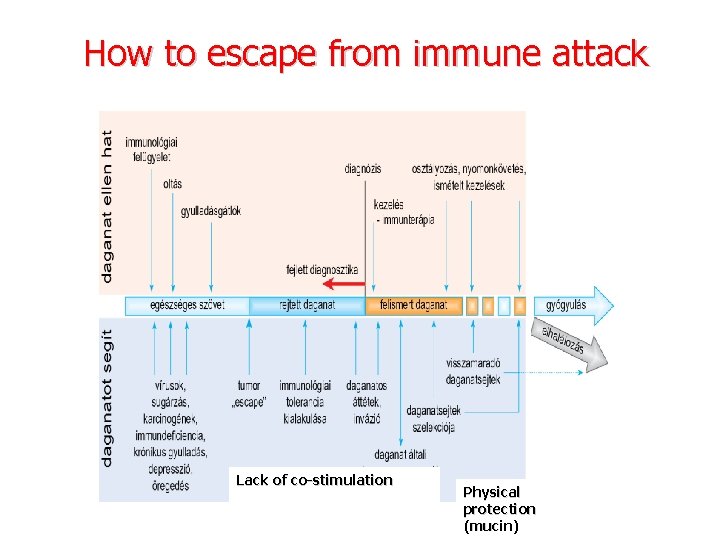
How to escape from immune attack Lack of co-stimulation Physical protection (mucin)

TUMOR ANTIGENS – elicit immune response Tumor Immunotherapy: Ølymphocyte dependent Øspecific Øanti tumor antibodies can be detected incease tumorspecific immune rsponse of the host Ømemory „Immunsurveilance” – to destroy tumor cells Øtumor specific antigens (TSA) – MHC bound peptides Øtumor associated antigens (TAA) - product of embrionic tissues 50 x- 100 x higher expression Isolation of tumor antigens: • c. DNS library - CTL clones, • by elution of peptides from surface of tumor cells by acidic elution

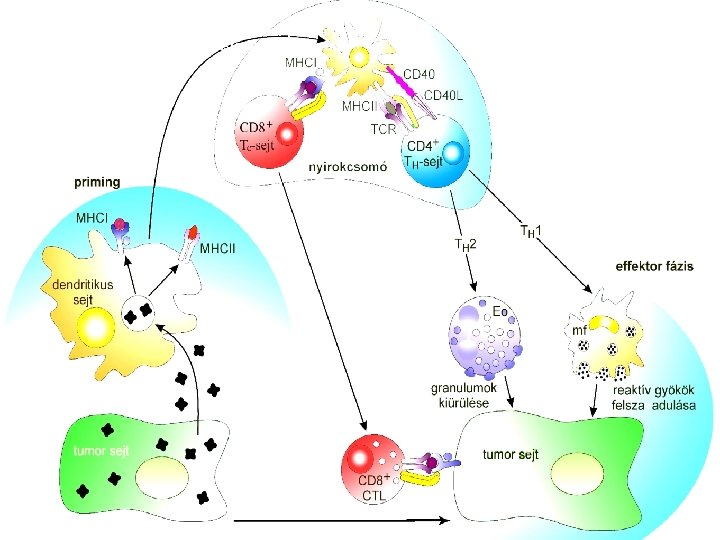
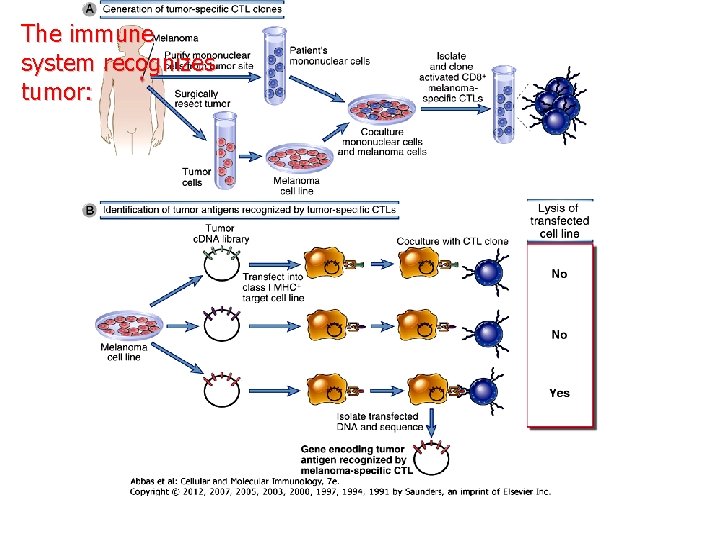
The immune system recognizes tumor:

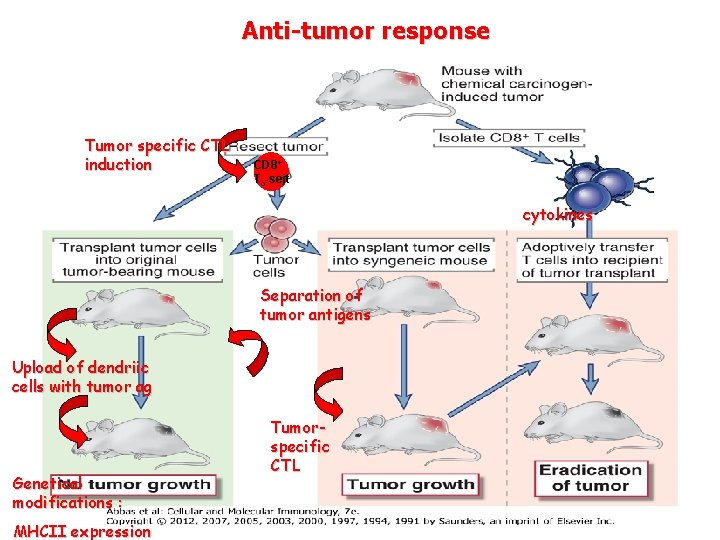
Anti-tumor response Tumor specific CTL induction CD 8+ Tc-sejt cytokines Separation of tumor antigens Upload of dendriic cells with tumor ag Genetical modifications : MHCII expression Tumorspecific CTL

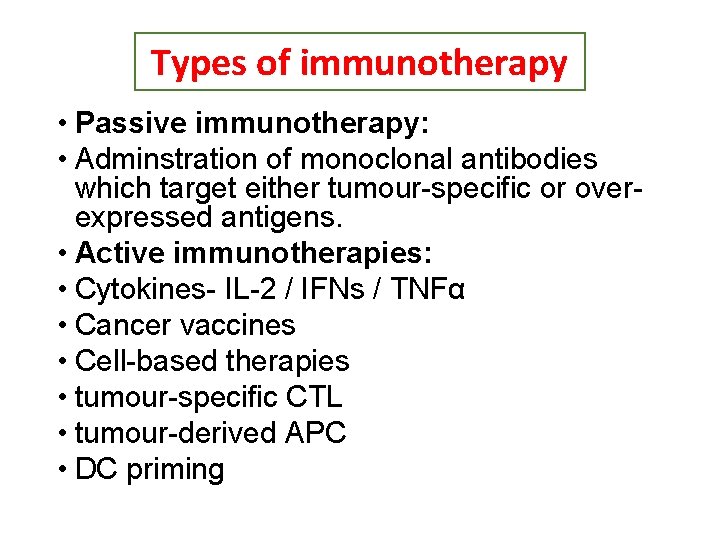
Types of immunotherapy • Passive immunotherapy: • Adminstration of monoclonal antibodies which target either tumour-specific or overexpressed antigens. • Active immunotherapies: • Cytokines- IL-2 / IFNs / TNFα • Cancer vaccines • Cell-based therapies • tumour-specific CTL • tumour-derived APC • DC priming

Current strategies in experimental immunotherapy of tumors

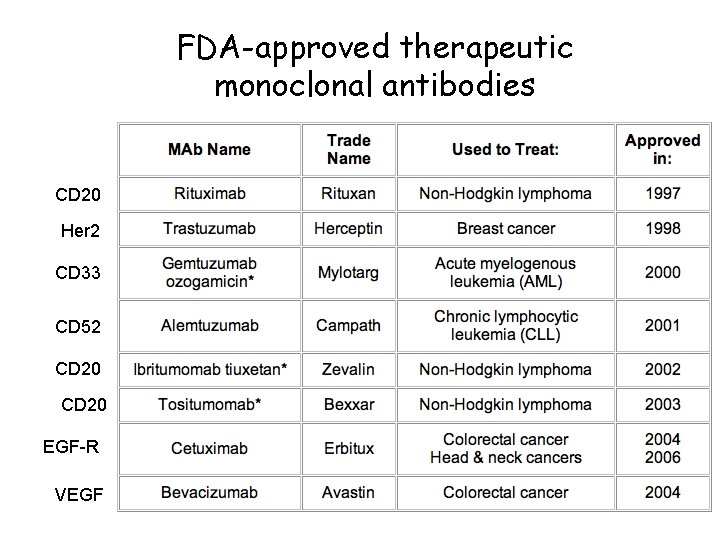
FDA-approved therapeutic monoclonal antibodies CD 20 Her 2 CD 33 CD 52 CD 20 EGF-R VEGF

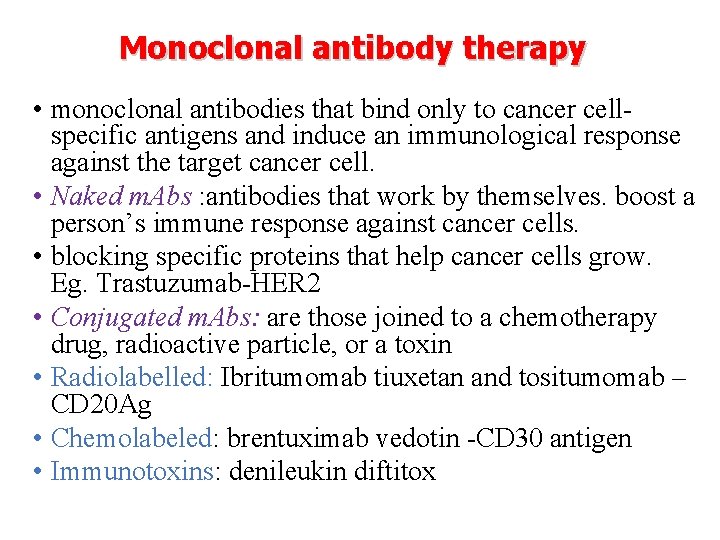
Monoclonal antibody therapy • monoclonal antibodies that bind only to cancer cellspecific antigens and induce an immunological response against the target cancer cell. • Naked m. Abs : antibodies that work by themselves. boost a person’s immune response against cancer cells. • blocking specific proteins that help cancer cells grow. Eg. Trastuzumab-HER 2 • Conjugated m. Abs: are those joined to a chemotherapy drug, radioactive particle, or a toxin • Radiolabelled: Ibritumomab tiuxetan and tositumomab – CD 20 Ag • Chemolabeled: brentuximab vedotin -CD 30 antigen • Immunotoxins: denileukin diftitox

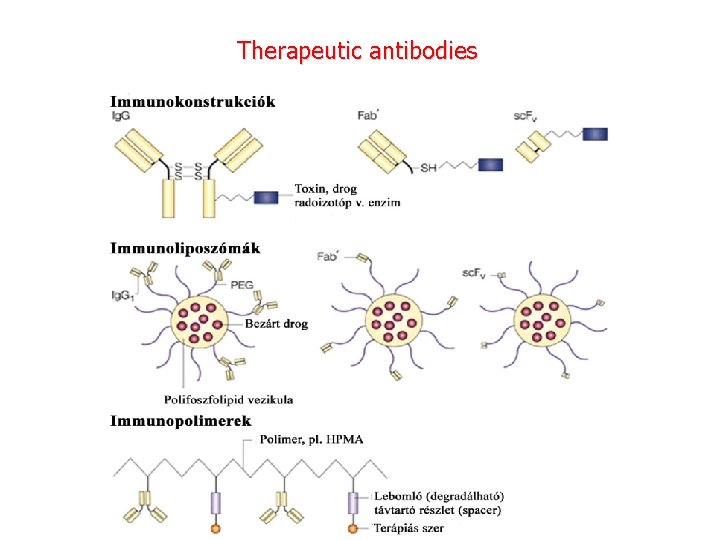
Therapeutic antibodies


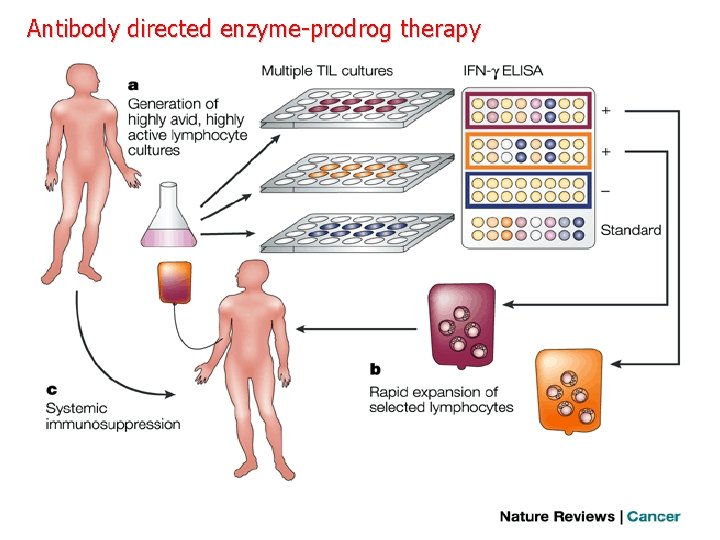
Monoclonal antibodies for cancer. ADEPT, antibody directed enzyme pro drug therapy; ADCC, antibody dependent cell-mediated cytotoxicity; CDC, complement dependent cytotoxicity; MAb, monoclonal antibody; sc. Fv single -chain Fv fragment


Antibody directed enzyme-prodrog therapy

Checkpoint antibodies Treatments that target PD-1 or PD-L 1 PD-1 is a checkpoint protein on immune cells called T cells. It normally acts as a type of “off switch” that helps keep the T cells from attacking other cells in the body. It does this when it attaches to PD-L 1, a protein on some normal (and cancer) cells. When PD-1 binds to PD-L 1, it basically tells the T cell to leave the other cell alone. Some cancer cells have large amounts of PD-L 1, which helps them evade immune attack. Monoclonal antibody treatments that target either PD-1 or PD-L 1 can boost the immune response against cancer cells and have shown a great deal of promise in treating certain cancers. Examples of treatments that target PD-1 include: Pembrolizumab (Keytruda®) Nivolumab (Opdivo®) Treatments that target CTLA-4 is another protein on some T cells that acts as a type of “off switch” to keep the immune system in check. Ipilimumab (Yervoy®) is a monoclonal antibody that attaches to CTLA-4 and stops it from working. This can boost the body’s immune response against cancer cells. This drug is used to treat melanoma of the skin. It is also being studied for use against other cancers. Because ipilimumab affects the immune system, it can sometimes cause serious or even lifethreatening side effects. In fact, compared to drugs that target PD-1 or PD-L 1, serious side effects seem to be more likely with ipilimumab.

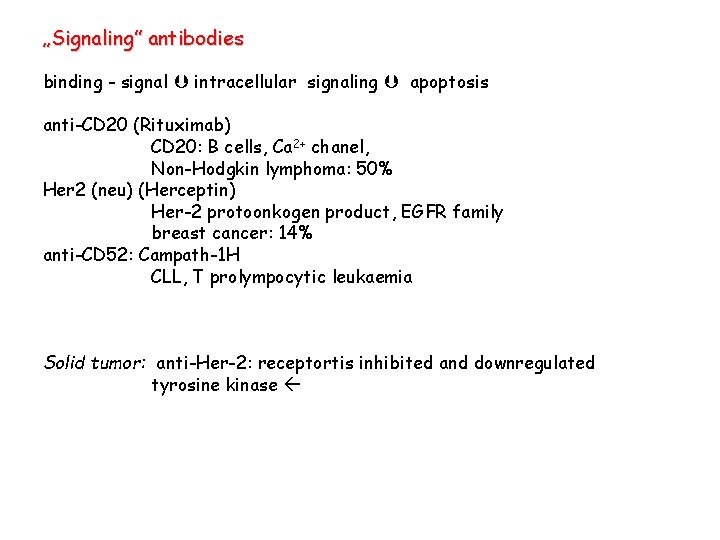
„Signaling” antibodies binding - signal intracellular signaling apoptosis anti-CD 20 (Rituximab) CD 20: B cells, Ca 2+ chanel, Non-Hodgkin lymphoma: 50% Her 2 (neu) (Herceptin) Her-2 protoonkogen product, EGFR family breast cancer: 14% anti-CD 52: Campath-1 H CLL, T prolympocytic leukaemia Solid tumor: anti-Her-2: receptortis inhibited and downregulated tyrosine kinase

Cytokine based cancer therapy

Cancer vaccines • Tumor cell vaccines: made from actual cancer cells that have been removed during surgery. • Antigen vaccines: These vaccines boost the immune system by using only one antigen rather than whole tumor cells • Dendritic cell vaccines: special immune cells in the body that help the immune system recognize cancer cells • DNA vaccines: Vectors can be given bits of DNA that code for protein antigens. • When the vectors are then injected into the body, this DNA might be taken up by cells and can instruct them to make specific antigens

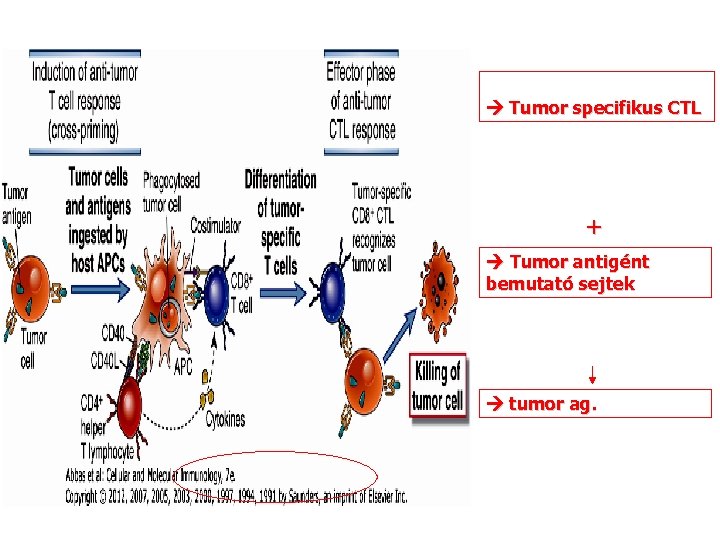
Tumor specifikus CTL + Tumor antigént bemutató sejtek tumor ag.

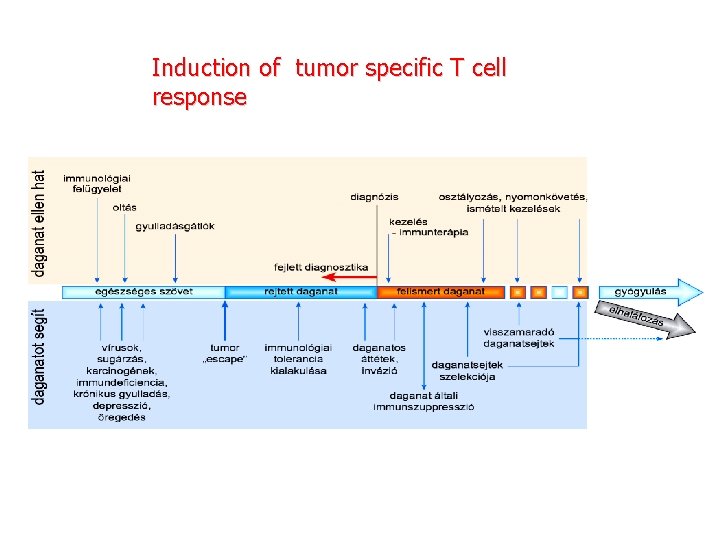
Induction of tumor specific T cell response

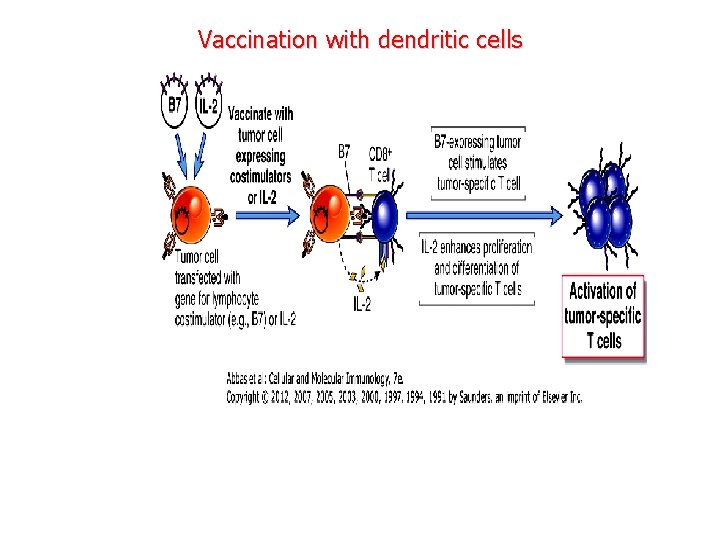
Vaccination with dendritic cells

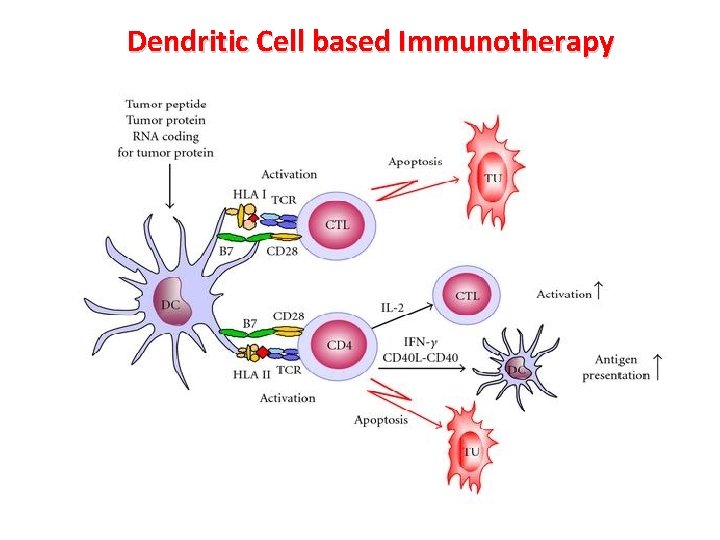
Dendritic Cell based Immunotherapy

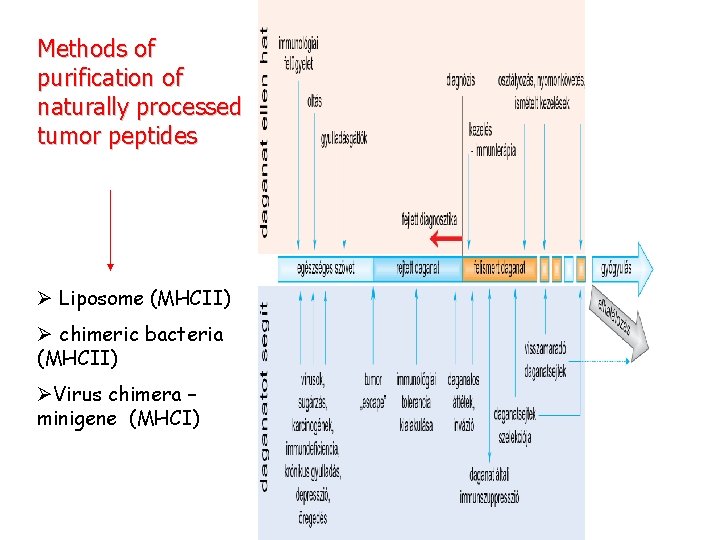
Methods of purification of naturally processed tumor peptides Ø Liposome (MHCII) Ø chimeric bacteria (MHCII) ØVirus chimera – minigene (MHCI)

Therapeutical application of peptides Synthetic peptides Native peptides advantages Specificity of the response mixture of many antigens unlimited patients can be used in many Homogeneous, pure activated CD 4, CD 8+T cells are Can be controlled by a few cells CD 4, CD 8 T activation disadvantages No tumor specificity, needed many tumor cells are For a few patients only, low concentration Mutant cells may escape, autoimmunity?

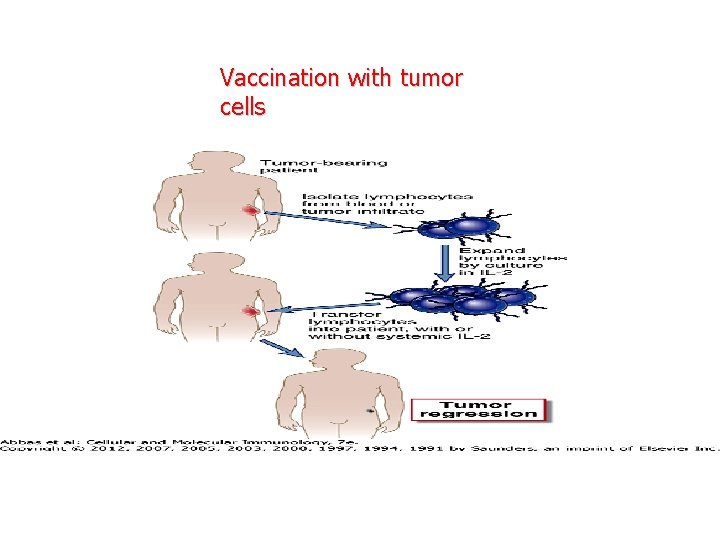
Vaccination with tumor cells


Therapeutic cancer vaccines Pramod K Srivastava The immunological bases of current approaches to therapeutic cancer vaccination (or ‘vacci-treatment’) have been established for a decade or longer. The new developments lie mostly in the lessons learnt from clinical testing of these approaches. Three lessons are particularly worthy of note. First, recently completed randomized Phase 3 trials suggest that vacci-treatment with autologous dendritic cells expressing prostatic acid phosphatase (for prostate cancer) or with cancer) autologous tumor-derived heat shock protein (gp 96)–peptide complexes show promise in enhancing survival of cancer complexes patients. These two approaches are undergoing further randomized clinical testing. Second, immunological monitoring of many clinical trials has failed to identify a surrogate marker for clinical outcomes. Finally, an increasing volume of literature suggests that protective immunity to human cancers is elicited by the mutated antigenic repertoire unique to each cancer.

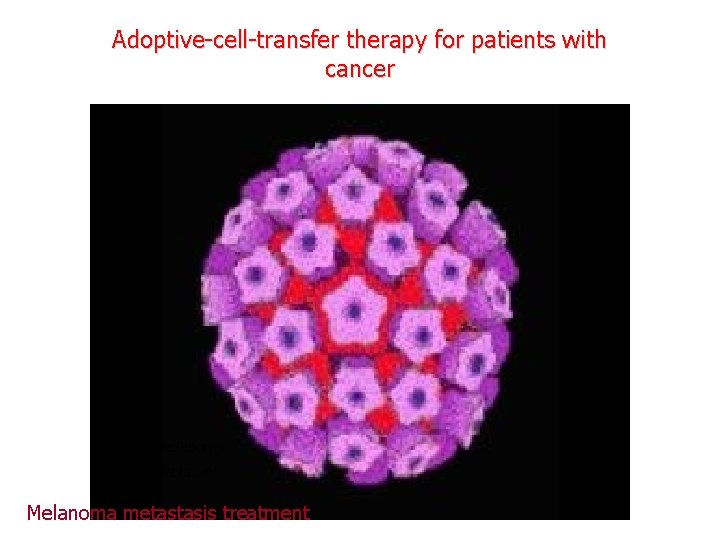
Adoptive-cell-transfer therapy for patients with cancer Limfocita depletion ”non-myeloablative” Melanoma metastasis treatment

Vaccines to protect against HPV infection and the subsequent risk of cervical cancer have recently become available, based on virus-like particle (VLP) technology. VLPs are produced by expressing the capsid proteins of the virus using recombinant DNA technology. When expressed in eukaryotic cells, the L 1 major capsid protein self-assembles into 360 -mer particles that physically and immunologically resemble the native virion. These highly immunogenic particles can, when administered as a vaccine with alum based adjuvants, protect not only against infection with the HPV types incorporated in the vaccine and some cross-reactive HPV types but also against the consequent premalignant disease Protection is durable over at least five years, and the vaccine appears to protect nearly 100% of immunised subjects. Human Papilloma Virus

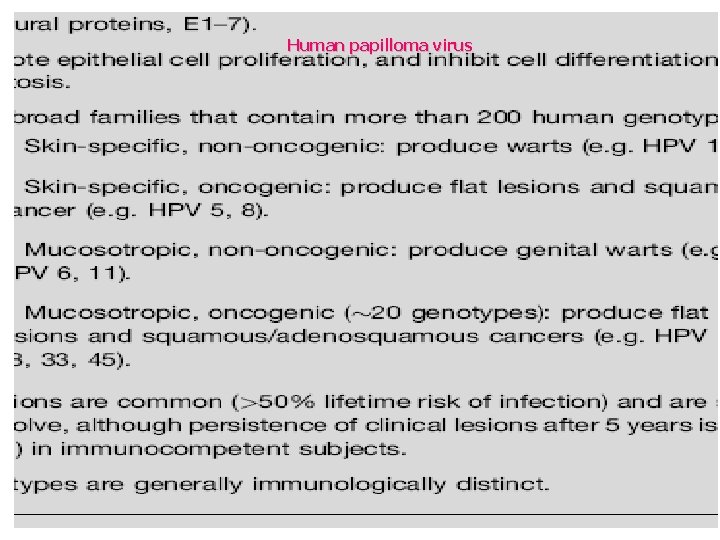
Human papilloma virus


Manipulation of antitumour immune responses by imm therapeutic vaccination. unog én Metastases that continue to grow are composed of tumour cells that lack antigens recognized by T cells and antibodies or are otherwise resistant to immune

Manipulation of antitumour immune responses by prophylactic vaccination. „high risk” indviduals Reactivation of tumor specific memory cells
 Difference between acquired immunity and innate immunity
Difference between acquired immunity and innate immunity Grammar to enrich and enhance writing
Grammar to enrich and enhance writing What are appetisers
What are appetisers Instinct definition psychology
Instinct definition psychology Objectives of social change
Objectives of social change A food or drink that stimulate the appetite
A food or drink that stimulate the appetite Bhag good to great
Bhag good to great Stimulate
Stimulate Define fashion merchandising
Define fashion merchandising Enhance an image
Enhance an image Cspnet
Cspnet Cosmetics are substances that are used to enhance
Cosmetics are substances that are used to enhance Enhance an image
Enhance an image Nyjc promotion criteria
Nyjc promotion criteria Enhance life
Enhance life Can hypnosis enhance recall of forgotten events
Can hypnosis enhance recall of forgotten events The difference between humoral and cell mediated immunity
The difference between humoral and cell mediated immunity Chapter 13 lymphatic system and immunity
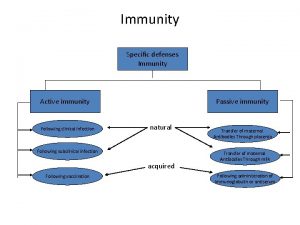
Chapter 13 lymphatic system and immunity Immunity classification chart
Immunity classification chart Chapter 16 lymphatic system and immunity
Chapter 16 lymphatic system and immunity Main components of lymphatic system
Main components of lymphatic system Difference between innate and learned behavior
Difference between innate and learned behavior Effector mechanism of humoral immunity
Effector mechanism of humoral immunity Nonspecific vs specific immunity
Nonspecific vs specific immunity Canra passed
Canra passed Adaptive noise immunity
Adaptive noise immunity Keva immunity booster benefits
Keva immunity booster benefits Assis. prof.
Assis. prof. Innate immunity examples
Innate immunity examples Conducted immunity test
Conducted immunity test Acquired immunity
Acquired immunity Acquired immunity definition
Acquired immunity definition Odibate
Odibate Active vs passive immunity
Active vs passive immunity Non specific immunity
Non specific immunity Neutrophil extracellular traps
Neutrophil extracellular traps Opsonization
Opsonization Cell mediated immunity
Cell mediated immunity Innate immunity first line of defense
Innate immunity first line of defense Innate immunity
Innate immunity Adaptative immunity
Adaptative immunity Immunity definition
Immunity definition Second line of defense immune system
Second line of defense immune system Passive immunity
Passive immunity Innate immunity first line of defense
Innate immunity first line of defense Innate immunity first line of defense
Innate immunity first line of defense Phagocytr
Phagocytr What is immunity
What is immunity Adaptive immunity
Adaptive immunity Conclusion of immunity
Conclusion of immunity Immunity assignment
Immunity assignment Cellular immune response
Cellular immune response Yasmeen taj
Yasmeen taj Innate immunity
Innate immunity What is immunity
What is immunity 3 lines of defense immunity
3 lines of defense immunity Local immunity
Local immunity Acquired immunity
Acquired immunity Humoral immunity
Humoral immunity Gastroenterology near galt
Gastroenterology near galt Exocytosis
Exocytosis