Tug of War Polarity and Electronegativity Think about

Tug of War Polarity and Electronegativity

Think about it • What does the word POLAR mean to you?

Polar Bear North/South Poles
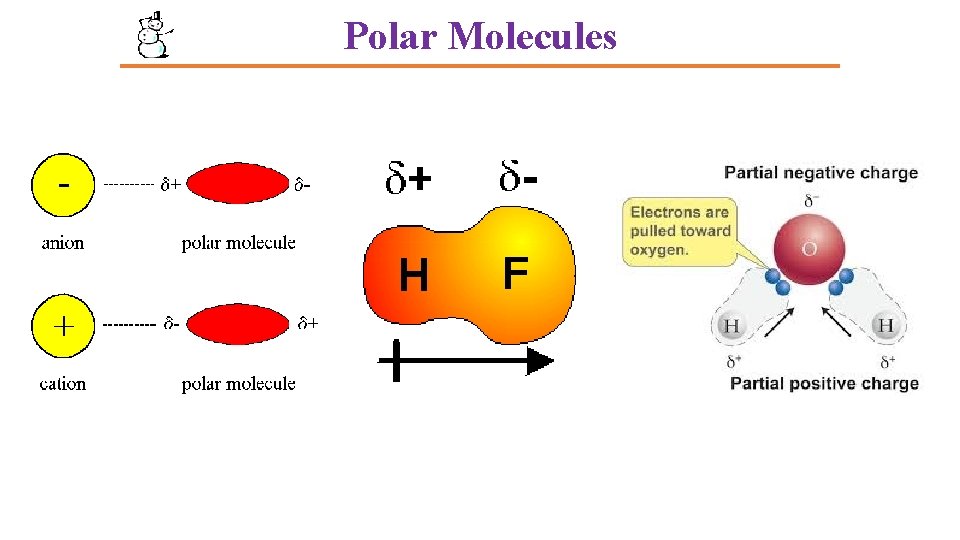
Polar Molecules

What is Electronegativity? Electronegativity: • How much an atom in a molecule attracts electrons
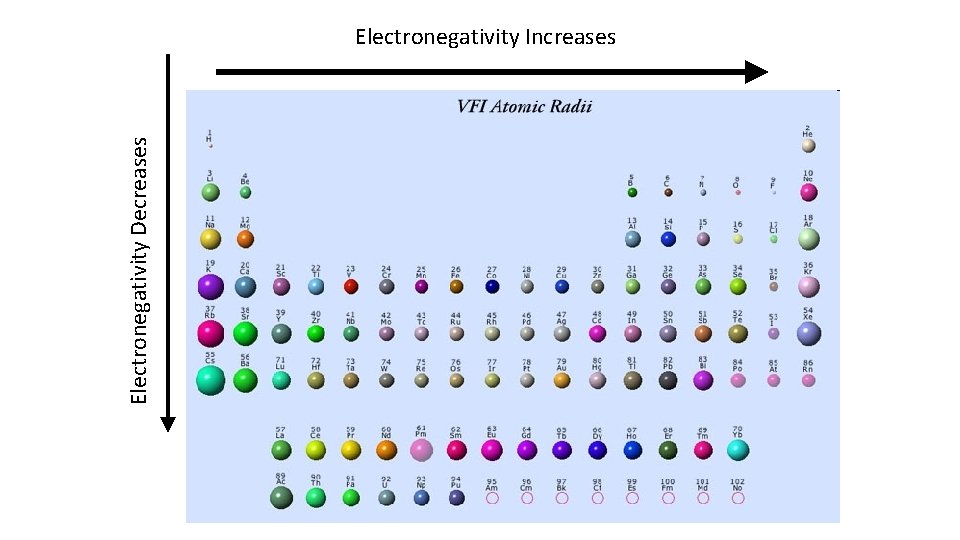
Electronegativity Decreases Electronegativity Increases
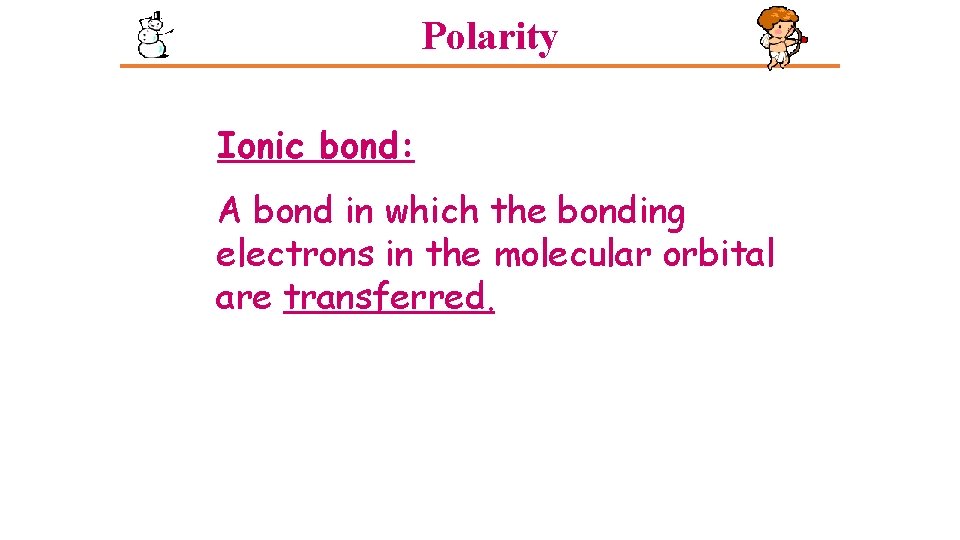
Polarity Ionic bond: A bond in which the bonding electrons in the molecular orbital are transferred.
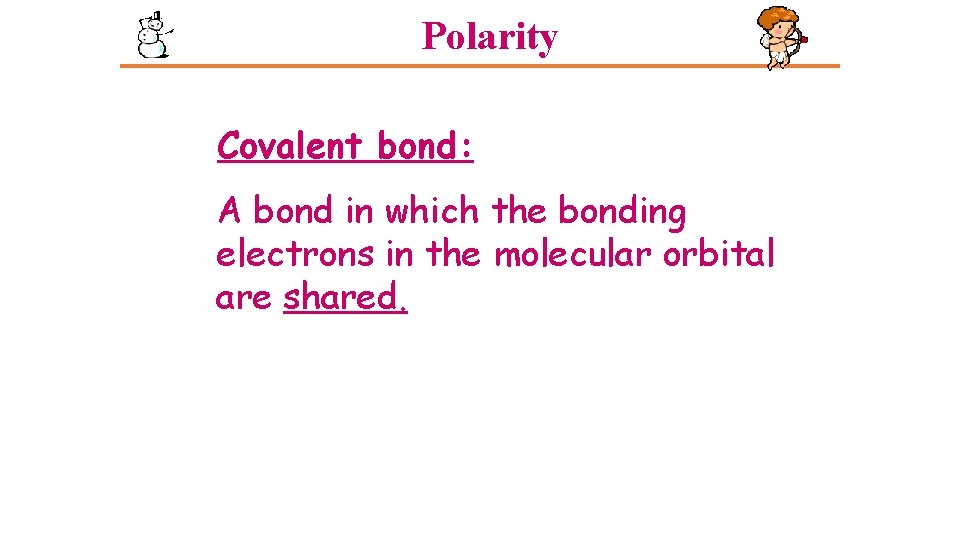
Polarity Covalent bond: A bond in which the bonding electrons in the molecular orbital are shared.
Polarity Nonpolar covalent bond: A covalent bond in which the bonding electrons in the molecular orbital are SHARED EQUALLY
Polarity Polar covalent bond: A covalent bond in which the bonding electrons in the molecular orbital are NOT SHARED EQUALLY
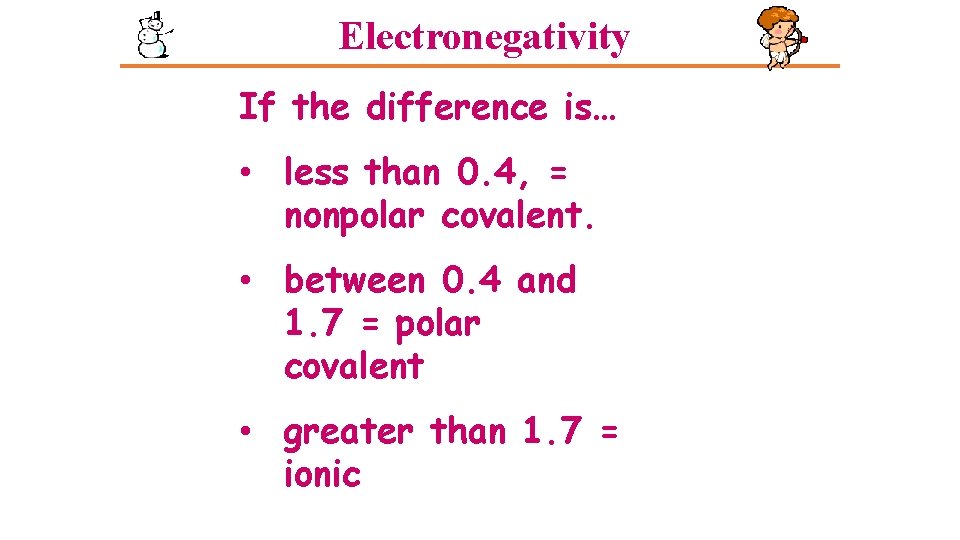
Electronegativity If the difference is… • less than 0. 4, = nonpolar covalent. • between 0. 4 and 1. 7 = polar covalent • greater than 1. 7 = ionic
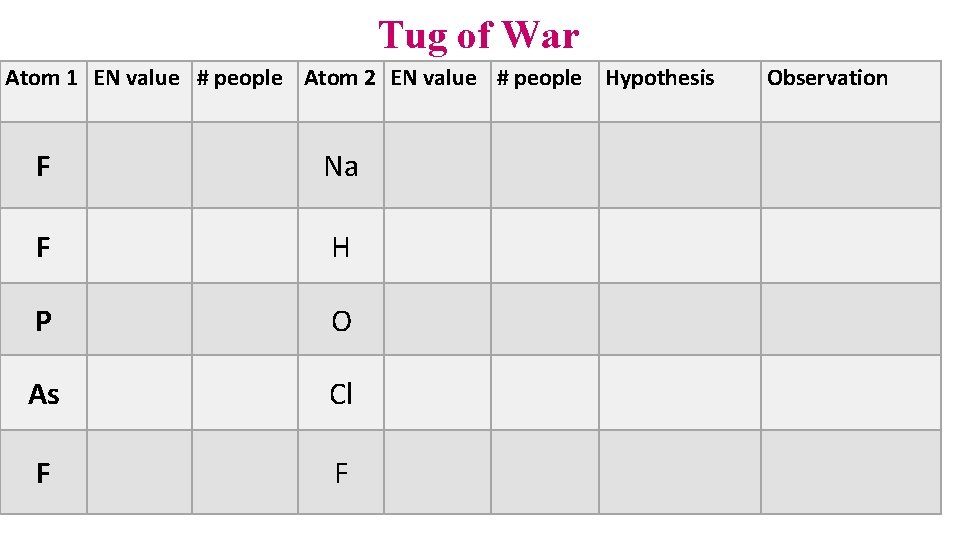
Tug of War Atom 1 EN value # people Atom 2 EN value # people Hypothesis F Na F H P O As Cl F F Observation
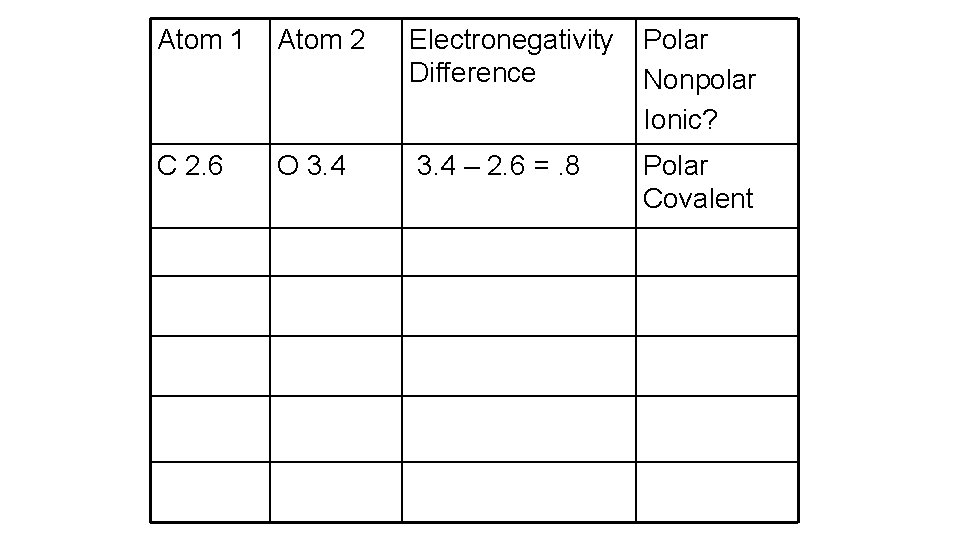
Atom 1 Atom 2 Electronegativity Difference Polar Nonpolar Ionic? C 2. 6 O 3. 4 – 2. 6 =. 8 Polar Covalent

Agenda • Unit 6 Topic 6 (Polarity) notes • Forensics Lab! Who kidnapped Ms. O ? ?

February 15, 2008 • What are the separation techniques that you know about?

February 19, 2008 • Which of the following sets will dissolve in each other? • Polar / Ionic • Non Polar / Ionic • Polar / Non Polar • Non Polar / Non Polar Homework: Read Electronegativity Pgs 168 -169 Take NOTES on what you read.

February 19, 2008 • Which of the following sets will dissolve in each other? • Polar / Ionic • Non Polar / Ionic • Polar / Non Polar • Non Polar / Non Polar
- Slides: 17