Trilogy Chemistry CHAPTER 7 Hydrocarbons Label the diagram
Trilogy – Chemistry – CHAPTER 7 – Hydrocarbons Label the diagram below to show the fractional distillation of crude oil. Define alkane and alkene. Draw diagrams to show the structure of these molecules. Discuss how crude oil is separated by boiling points. KEY WORDS: hydrocarbon crude oil viscosity volatile fractional distillation boiling point cracking ASSESSMENT:
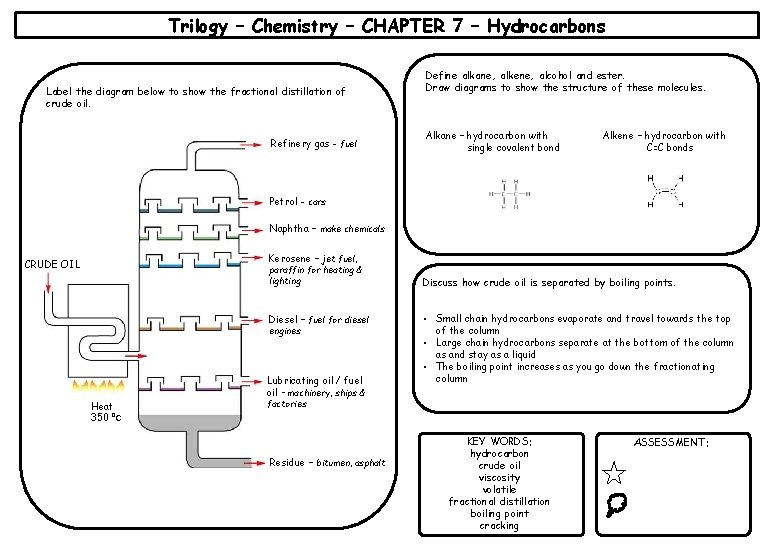
Trilogy – Chemistry – CHAPTER 7 – Hydrocarbons Label the diagram below to show the fractional distillation of crude oil. Refinery gas - fuel Define alkane, alkene, alcohol and ester. Draw diagrams to show the structure of these molecules. Alkane – hydrocarbon with single covalent bond Alkene – hydrocarbon with C=C bonds Petrol - cars Naphtha – make chemicals Kerosene – jet fuel, CRUDE OIL paraffin for heating & lighting Diesel – fuel for diesel engines Heat 350 ⁰C Lubricating oil / fuel oil – machinery, ships & Discuss how crude oil is separated by boiling points. • Small chain hydrocarbons evaporate and travel towards the top of the column • Large chain hydrocarbons separate at the bottom of the column as and stay as a liquid • The boiling point increases as you go down the fractionating column factories Residue – bitumen, asphalt KEY WORDS: hydrocarbon crude oil viscosity volatile fractional distillation boiling point cracking ASSESSMENT:
- Slides: 2