Tridentate Binding Structure of Cu2 2 6 2

Tridentate Binding Structure of Cu-(2, 2 : 6 , 2 -Terpyridine) XU WANG and DONG-SHENG YANG Department of Chemistry, University of Kentucky, Lexington, KY, 40506

Motivations FTerpyridine: u One of the most used ligands for construction of supramolecular systems; u Important ligand in testing catalytic activity, coordination chemistry, organometallic and photochemistry; u High binding affinity towards transition metal. FMetal complexes: u u Polymers, surface; Electronic and optical properties: solar cell, nanodevices…
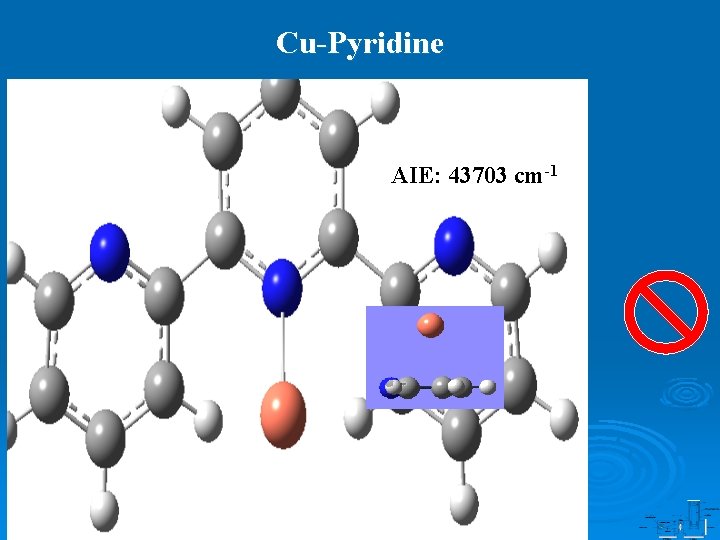
Cu-Pyridine AIE: 43703 cm-1
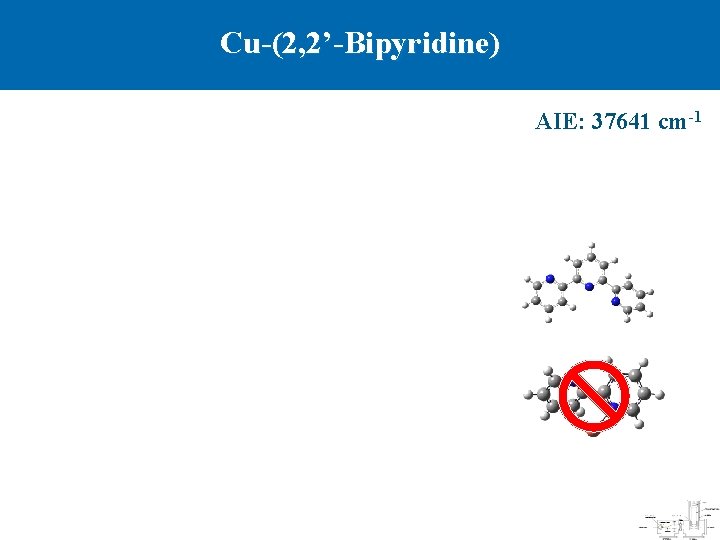
Cu-(2, 2’-Bipyridine) AIE: 37641 cm-1
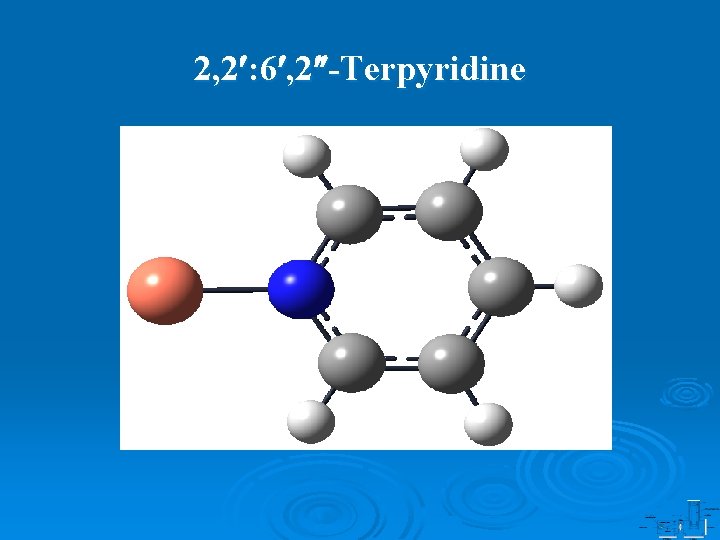
2, 2 : 6 , 2 -Terpyridine

Methodology Ø Laser ablation Ø Time-of-flight mass spectrometry (TOF-MS) Ø Photoionization efficiency (PIE) spectroscopy Ø Zero electron kinetic energy (ZEKE) photoelectron Spectroscopy Ø Calculations: B 3 LYP/6 -311+G(d, p) Ø Simulations: Franck-Condon and harmonic approximations
Schematic Diagram of PFI-ZEKE Apparatus
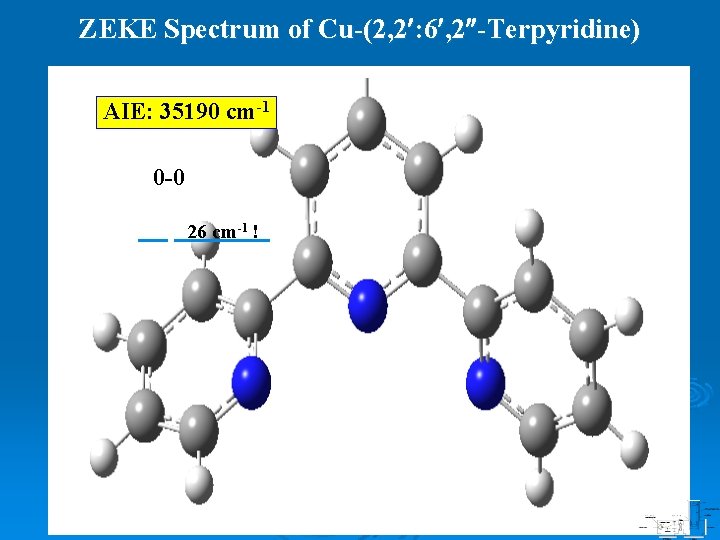
ZEKE Spectrum of Cu-(2, 2 : 6 , 2 -Terpyridine) AIE: 35190 cm-1 0 -0 26 cm-1 !
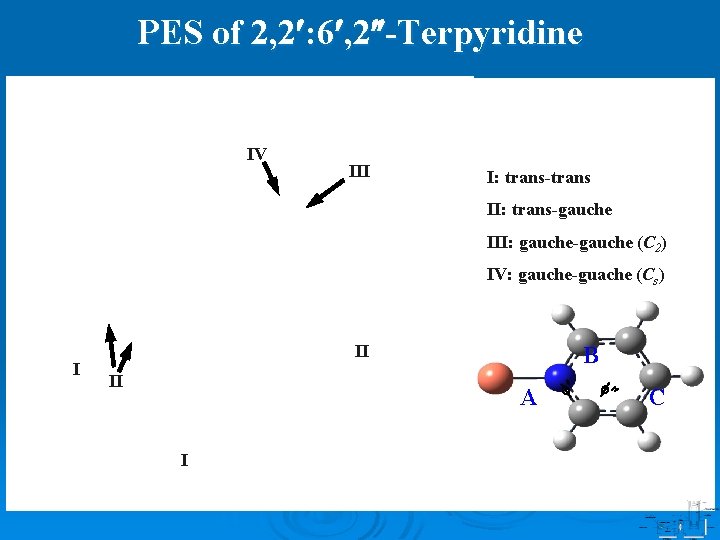
PES of 2, 2 : 6 , 2 -Terpyridine IV III I: trans-trans II: trans-gauche III: gauche-gauche (C 2) IV: gauche-guache (Cs) I II II B A I C
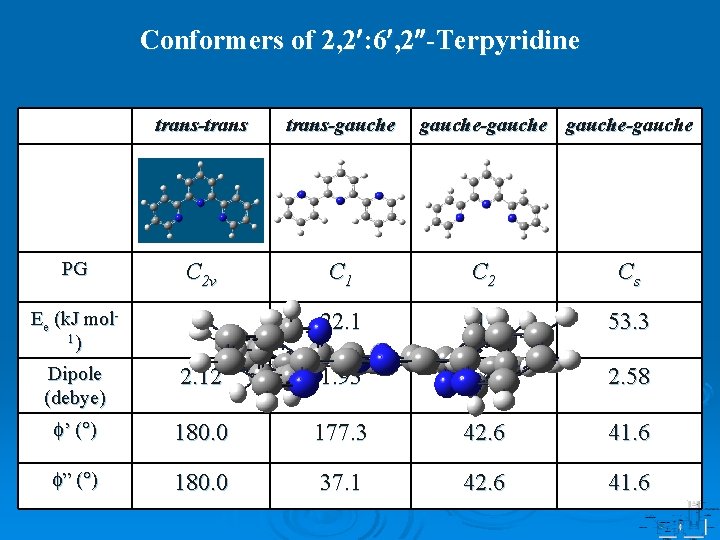
Conformers of 2, 2 : 6 , 2 -Terpyridine trans-trans-gauche-gauche PG C 2 v C 1 C 2 Cs Ee (k. J mol 1) 0 22. 1 51. 5 53. 3 Dipole (debye) 2. 12 1. 93 3. 42 2. 58 ’ ( ) 180. 0 177. 3 42. 6 41. 6 ” ( ) 180. 0 37. 1 42. 6 41. 6
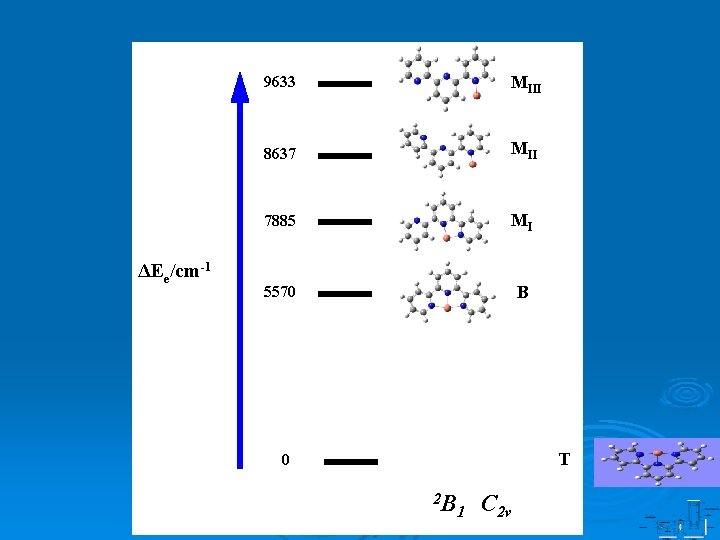
ΔEe/cm-1 9633 MIII 8637 MII 7885 MI 5570 B T 0 2 B 1 C 2 v
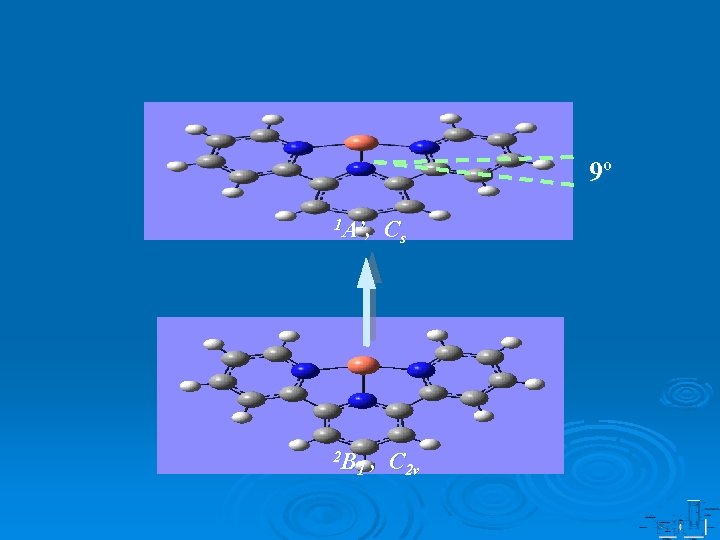
9º 1 A’, 2 B 1 Cs , C 2 v

Experimental and Simulated Spectra of Five Isomers ZEKE B MI MIII
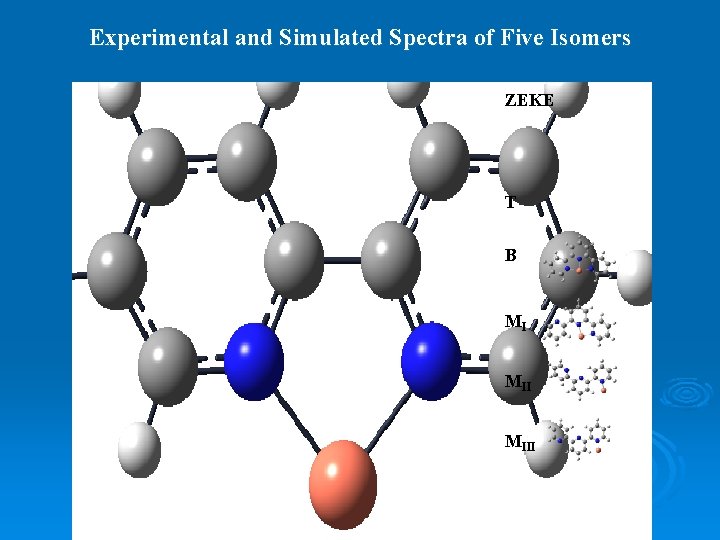
Experimental and Simulated Spectra of Five Isomers ZEKE T B MI MIII
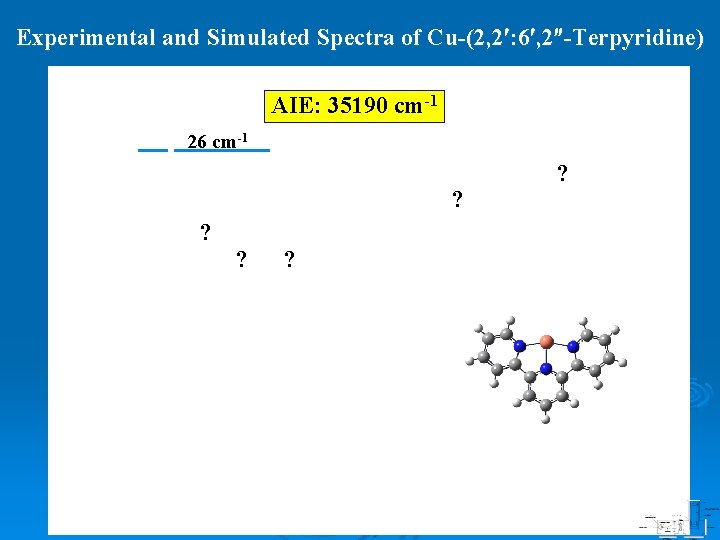
Experimental and Simulated Spectra of Cu-(2, 2 : 6 , 2 -Terpyridine) AIE: 35190 cm-1 26 cm-1 ? ? ? ? ?
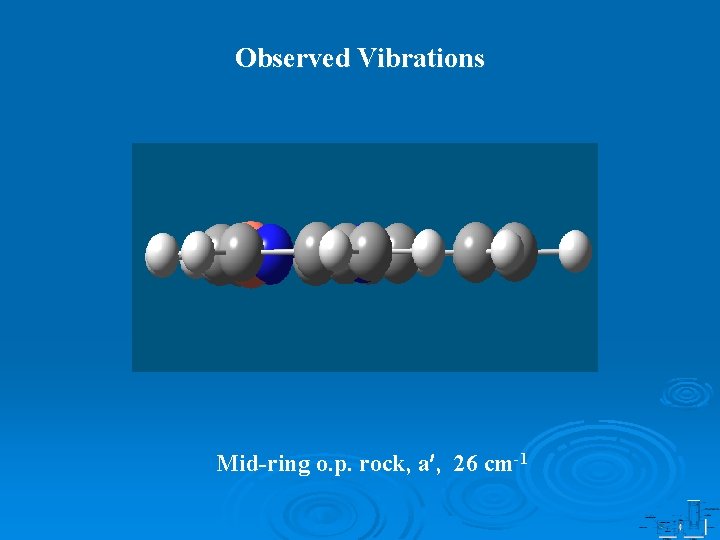
Observed Vibrations Mid-ring o. p. rock, a , 26 cm-1
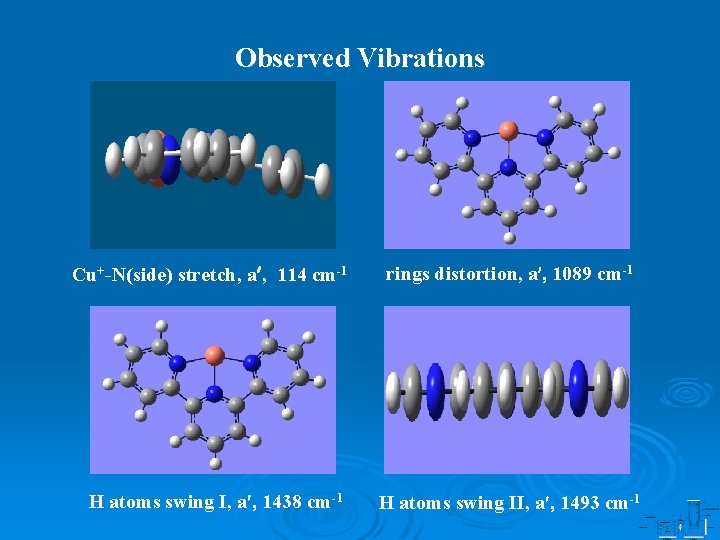
Observed Vibrations Cu+-N(side) stretch, a , 114 cm-1 rings distortion, a , 1089 cm-1 H atoms swing I, a , 1438 cm-1 H atoms swing II, a , 1493 cm-1
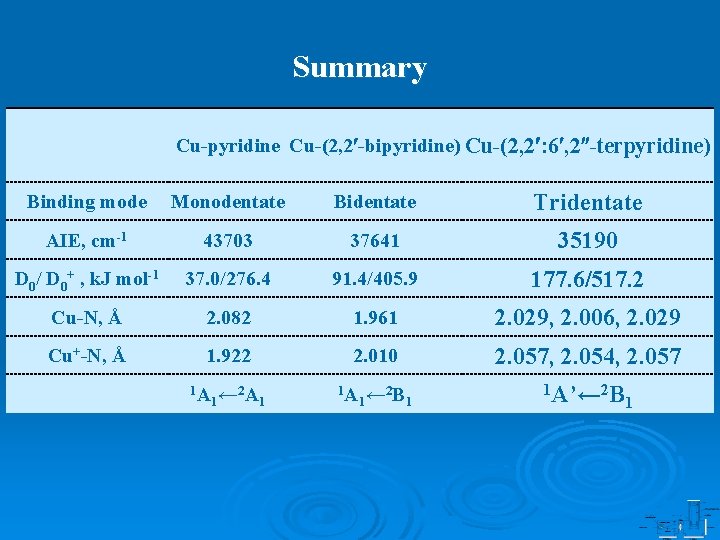
Summary Cu-pyridine Cu-(2, 2 -bipyridine) Cu-(2, 2 : 6 , 2 -terpyridine) Binding mode Monodentate Bidentate Tridentate AIE, cm-1 43703 37641 35190 D 0/ D 0+ , k. J mol-1 37. 0/276. 4 91. 4/405. 9 177. 6/517. 2 Cu-N, Å 2. 082 1. 961 2. 029, 2. 006, 2. 029 Cu+-N, Å 1. 922 2. 010 2. 057, 2. 054, 2. 057 1 A 1← 2 A 1 1 A 1← 2 B 1 1 A’← 2 B 1

To be continued…
- Slides: 19