Trial Design Considerations for Percutaneous Mitral Valve Repair




- Slides: 11

Trial Design Considerations for Percutaneous Mitral Valve Repair Laura Mauri, MD, MSc Brigham and Women’s Hospital Harvard Medical School

Laura Mauri, MD § Honoraria – – Abbott Laboratories Cordis Corporation Boston Scientific Corporation Medtronic, Inc.

Trial Design Considerations for Percutaneous Mitral Valve Repair • Standard of Care • Patient Population • Expected Benefits and Risks of Percutaneous Repair 3

Trial Design Considerations for Percutaneous Mitral Valve Repair Standard of Care varies by the type of MR • Spectrum of Mitral Regurgitation: • Asymptomatic • Symptomatic • Early left ventricular dysfunction • Range of mechanisms • Degenerative – leaflet pathology • Functional – left ventricular enlargement • Repair preferred when feasible • Replacement may be performed when repair cannot reliably reduce MR • High risk MVR vs Medical therapy 4

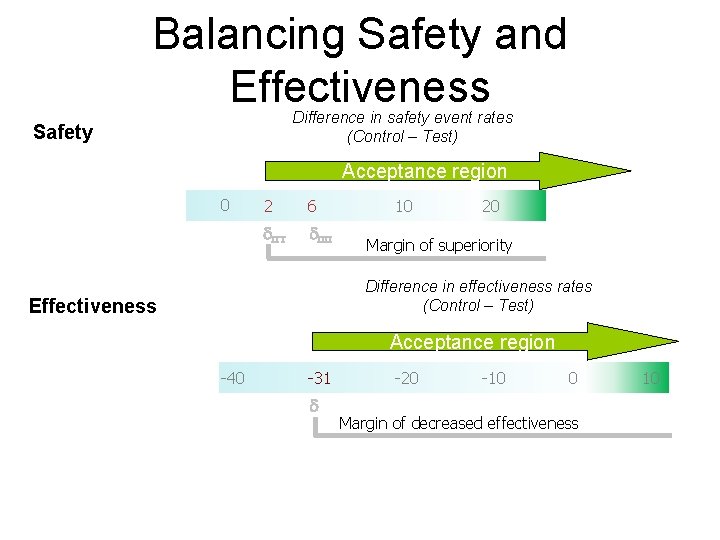
Trial Design Considerations for Percutaneous Mitral Valve Repair Compared with surgery, first in class therapy may not have same efficacy as the surgery • Tradeoff between invasiveness vs potential need for more than one procedure – similar to current tradeoff for PCI with CABG in complex CAD • For trial design, an acceptable margin of difference in safety and efficacy that would describe a reasonable balance needs to be prespecified • This margin should preserve some of the efficacy of surgery, but would not be expected to be the same • When trial is complete, the totality of results can be interpreted for clinical decision making – with actual rates rather than p values 5

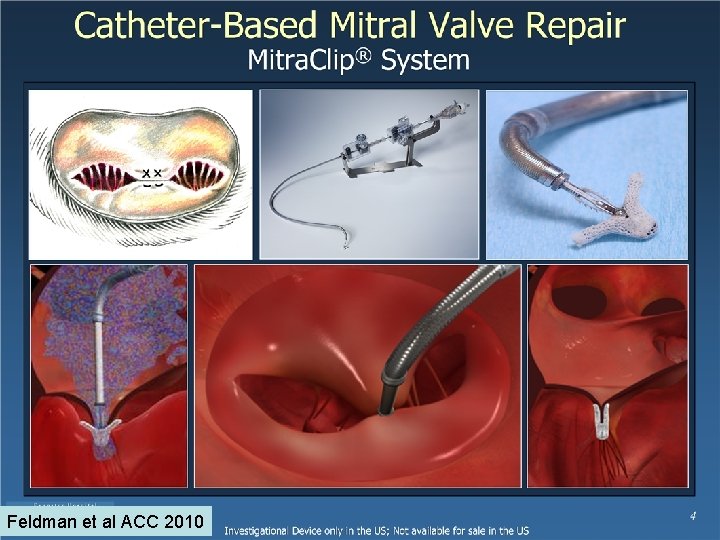
Feldman et al ACC 2010

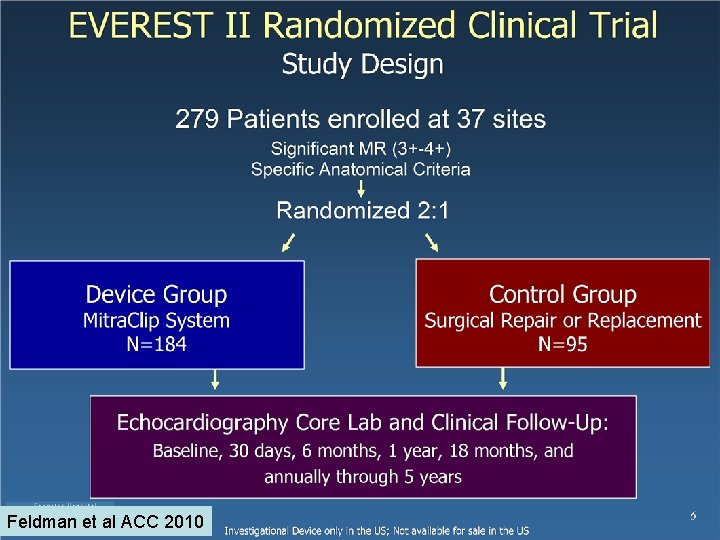
Feldman et al ACC 2010

Feldman et al ACC 2010

Trial Design Considerations for Percutaneous Mitral Valve Repair • Key Design Features of Everest II RCT of mitral valve surgery vs perctuaneous mitral repair • Randomization • Study population meeting ACC/ AHA criteria for MV surgery • Participation of interventionalist, surgeon, echocardiographer as investigators • Central adjudication of endpoints • Core laboratory to assess mitral regurgitation severity • Safety – 30 day Major Adverse Events • Efficacy – 1 year Freedom from death, mitral valve surgery and MR grade 3 or 4+ • Left ventricular dimensions, quality of life • 5 year follow-up 9

Balancing Safety and Effectiveness Difference in safety event rates (Control – Test) Safety Acceptance region 0 2 6 d. ITT d. PP 10 20 Margin of superiority Difference in effectiveness rates (Control – Test) Effectiveness Acceptance region -40 -31 d -20 -10 0 Margin of decreased effectiveness 10

Trial Design Considerations for Percutaneous Mitral Valve Repair • Everest II met prespecifed analyses of efficacy and safety • Randomization was the key feature to allow clinical interpretation of results without bias • Results of Everest II, ITT analysis showed that there was tradeoff between effectiveness and safety • ~20% of subjects assigned to percutaneous treatment required subsequent surgery for MR • Surgery as a first procedure was more effective (MR grade was less) • Percutaneous was associated with fewer complications • Left ventricular dimensions improved for both treatment arms • Selection of a percutaneous strategy may allow some patients to avoid surgery by reducing MR and preserving option for future surgery • 2 year follow up to be presented at ACC 11