Trends in the Periodic Table IDENTIFYING THE PATTERNS

Trends in the Periodic Table IDENTIFYING THE PATTERNS

What we will investigate Atomic Ø size how big the atoms are Ionization Ø How easy it is to remove an electron from an atom Electron Ø Affinity How easily an atom will gain an electron Ionic Ø energy size How big ions are

Key ideas Like charges repel while unlike charges attract. The larger the charge, the more it will either attract or repel. The closer in distance unlike charges are to each other, the greater the force of attraction between them while the farther they are apart, the weaker the force of attraction. Also, the closer in distance like charges are the greater the force of repulsion and while the farther they are apart, the weaker the force of repulsion.

Key ideas… Coulomb’s Law
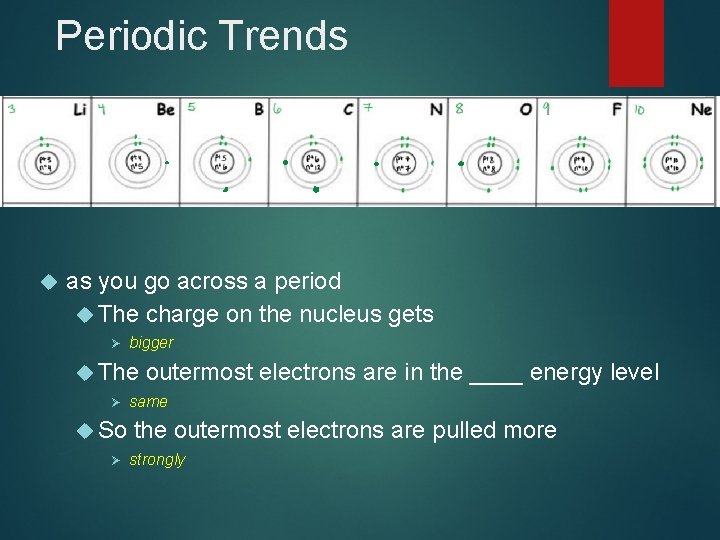
Periodic Trends as you go across a period The charge on the nucleus gets Ø bigger The Ø So Ø outermost electrons are in the ____ energy level same the outermost electrons are pulled more strongly
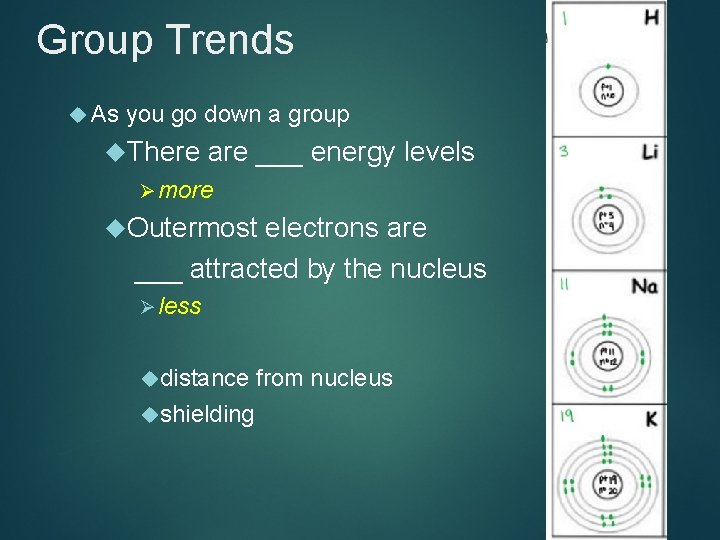
Group Trends As you go down a group There are ___ energy levels Ø more Outermost electrons are ___ attracted by the nucleus Ø less distance shielding from nucleus

Shielding: “Electrons in filled energy levels between the nucleus and outer shell electrons shield the outer shell electrons somewhat from the effect of protons in the nucleus. ” The electron on the outside energy level has to look through all the other energy levels (core electrons) to “see” the nucleus +

Shielding The electron on the outside energy level has to look through all the other energy levels (core electrons) to see the nucleus A second electron has the same shielding In the same energy level (period) the shielding is the same +

Shielding As the energy levels change, the shielding changes Lower down the group More energy levels ______ shielding Ømore + Outer electron _____ attracted Øless Three No shielding One Two shields

Effective Nuclear Charge Zeff The net force of attraction between the electrons and the nucleus of the atom Charge on nucleus, energy level of valence electrons and shielding of valence electrons by core electrons are considered Zeff of Cl > Zeff of Si Why? Ø More protons Ø Same energy level and same shielding
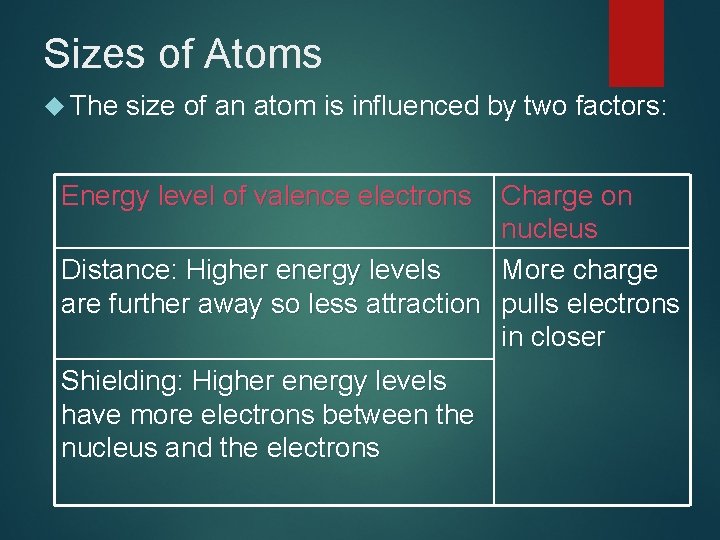
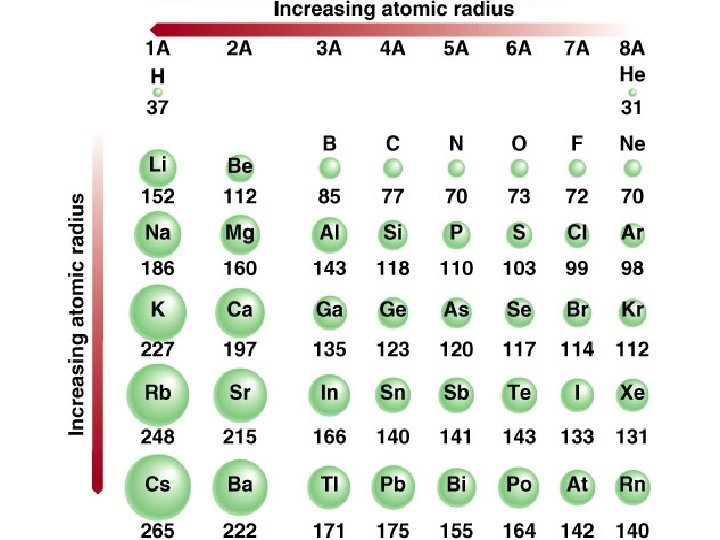
Sizes of Atoms The size of an atom is influenced by two factors: Energy level of valence electrons Charge on nucleus Distance: Higher energy levels More charge are further away so less attraction pulls electrons in closer Shielding: Higher energy levels have more electrons between the nucleus and the electrons

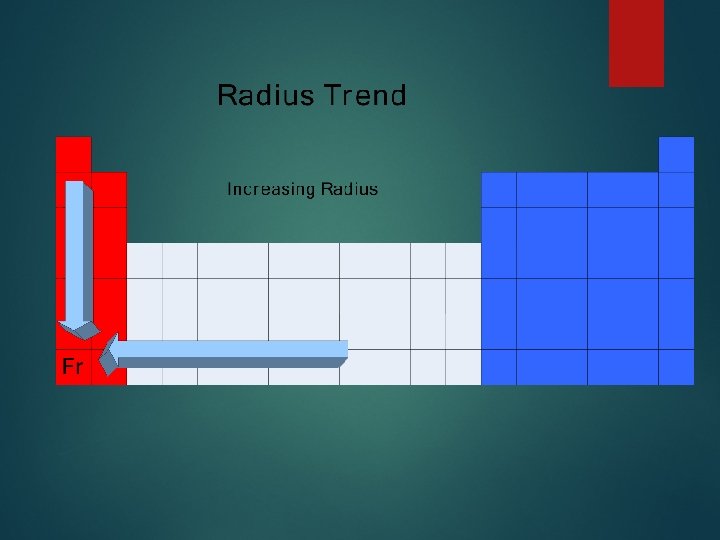
Group trends As we go down a group Each atom has another energy level Distance between valence electrons and nucleus is large so less attraction More So shielding so less attraction the atoms get bigger H Li Na K Rb
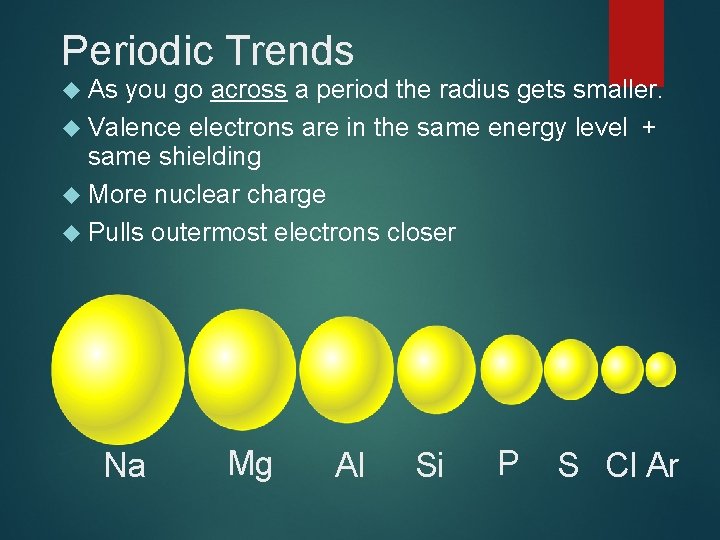
Periodic Trends As you go across a period the radius gets smaller. Valence electrons are in the same energy level + same shielding More nuclear charge Pulls outermost electrons closer Na Mg Al Si P S Cl Ar

Ionization Energy of Atoms The amount of energy required to completely remove an electron from a gaseous atom. Removing one electron makes a +1 ion Referred to as IE
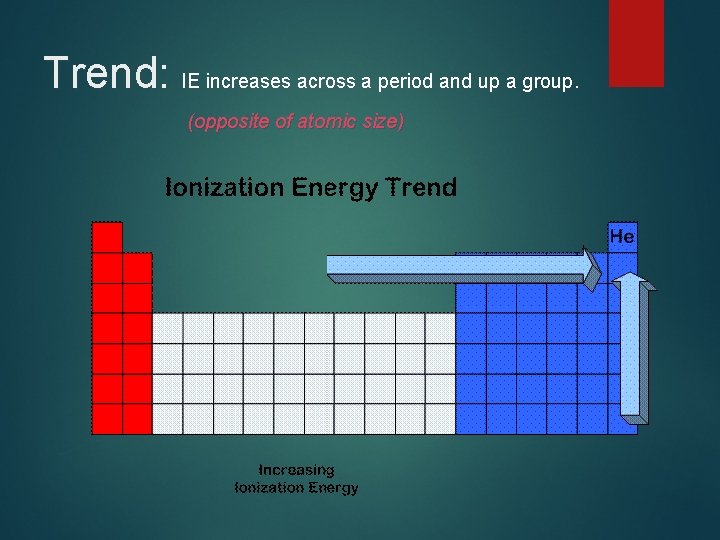
Trend: IE increases across a period and up a group. (opposite of atomic size)
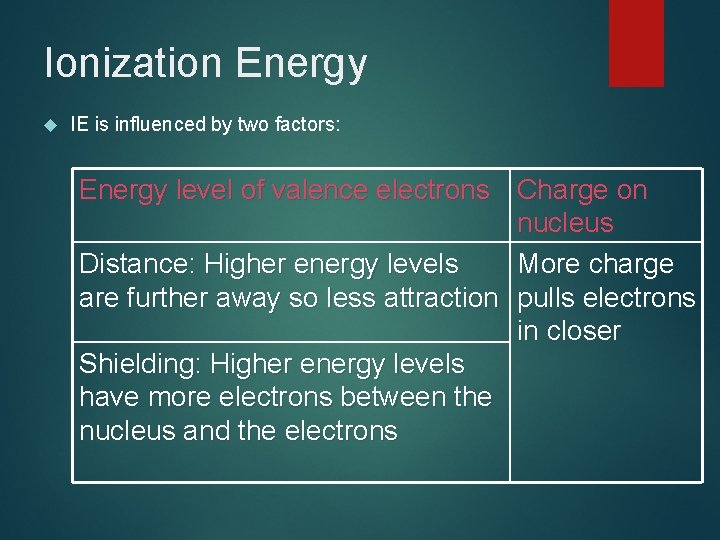
Ionization Energy IE is influenced by two factors: Energy level of valence electrons Charge on nucleus Distance: Higher energy levels More charge are further away so less attraction pulls electrons in closer Shielding: Higher energy levels have more electrons between the nucleus and the electrons

Periodic Trends As you go across a period the IE increases Valence electrons are in the same energy level + same shielding More nuclear charge The outermost electrons feel a stronger force of attraction to the nucleus It is more difficult to remove an electron Therefore it takes more energy to remove an electron

Group trends As we go up a group the IE increases Each atom has one less energy level Distance between valence electrons and nucleus gets smaller so more attraction Less shielding so more attraction It is harder to remove an electron So it takes more energy to remove an electron

Electron Affinity of Atoms (EA) The change in potential energy when an electron is added to a gaseous atom. ?

What EA is basically giving us: A measure of how attracted an electron is to an atom How much it would “like” to form a -1 anion
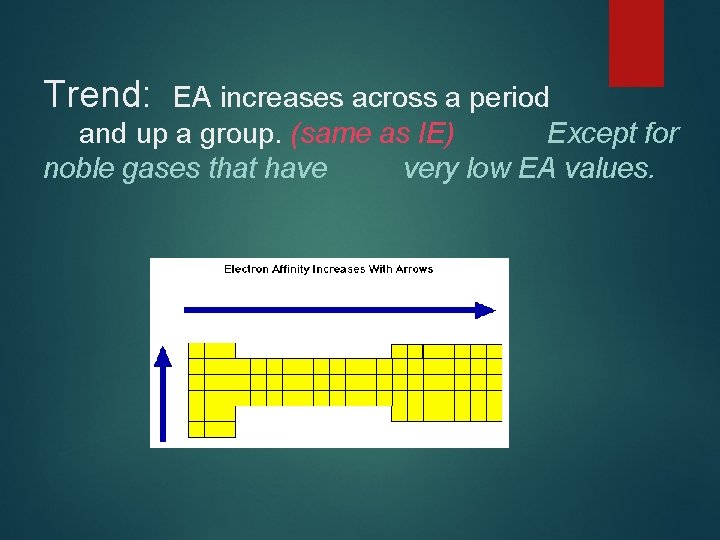
Trend: EA increases across a period and up a group. (same as IE) Except for noble gases that have very low EA values.
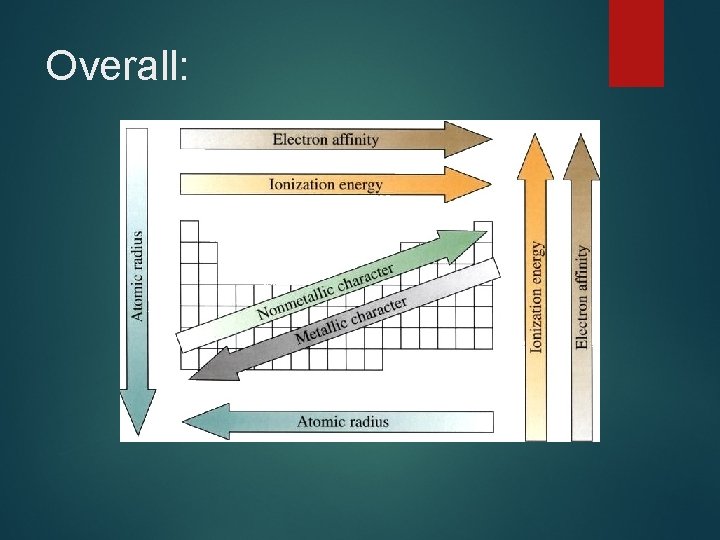
Overall:

Ionic Size: Cations are positiveions formed by losing electrons in order to have filled outer shells Metals form cations Cations from are smaller than the atom they come
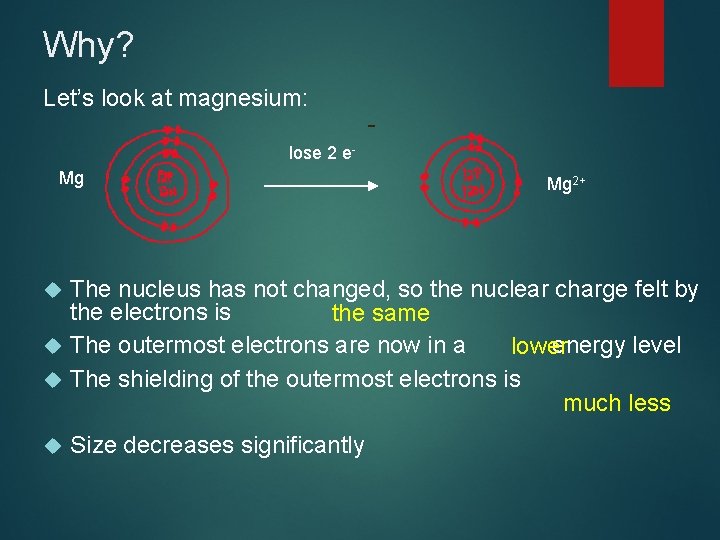
Why? Let’s look at magnesium: - lose 2 e. Mg Mg 2+ The nucleus has not changed, so the nuclear charge felt by the electrons is the same The outermost electrons are now in a energy level lower The shielding of the outermost electrons is much less Size decreases significantly

Ionic size: Anions are negativeions formed by gaining electrons in order to have filled outer shells Nonmetals Anions form anions are bigger than the atom they come from
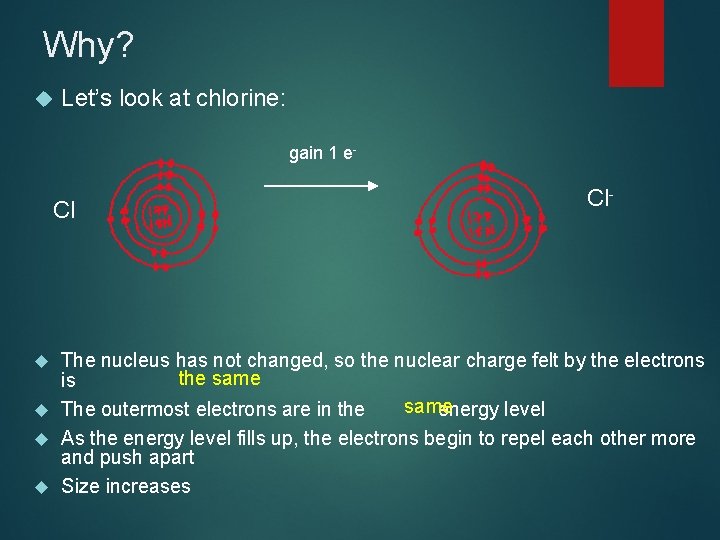
Why? Let’s look at chlorine: gain 1 e- Cl Cl- The nucleus has not changed, so the nuclear charge felt by the electrons the same is same The outermost electrons are in the energy level As the energy level fills up, the electrons begin to repel each other more and push apart Size increases
- Slides: 28