Trends in the Periodic Table CHAPTER 7 Increasing

Trends in the Periodic Table CHAPTER 7

Increasing reactivity with water (group 1): http: //youtu. be/uixx. Jt. JPVXk Increasing reactivity with water (Braniac): http: //youtu. be/m 55 kgy. Ap. Yr. Y
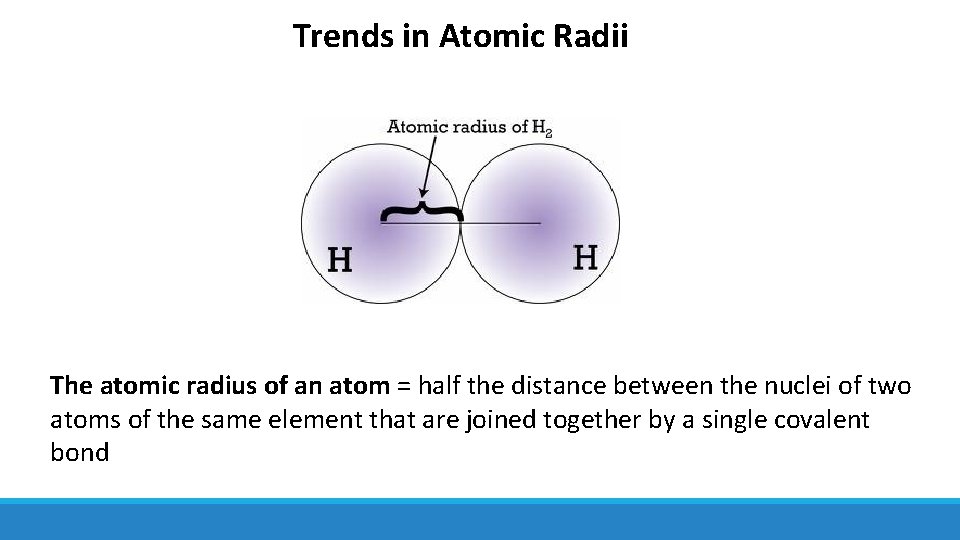
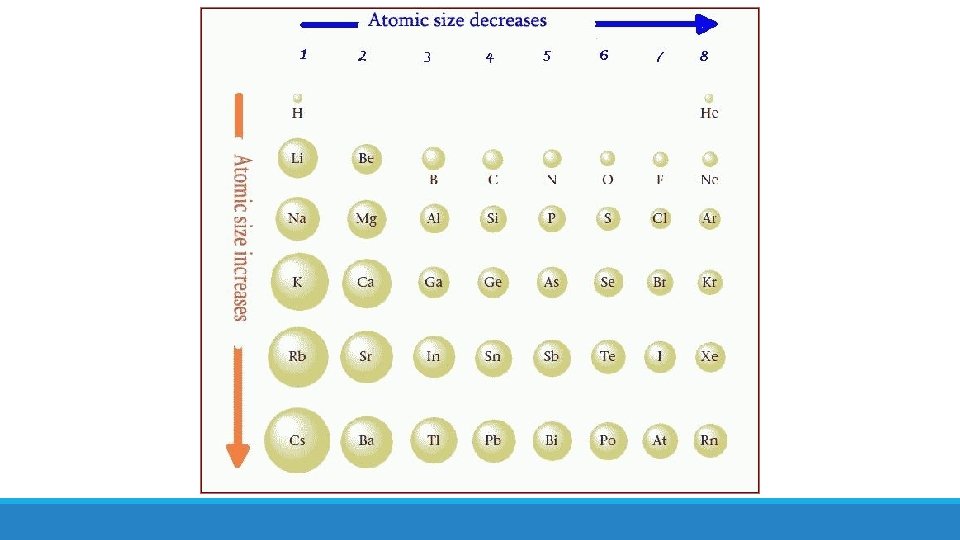
Trends in Atomic Radii The atomic radius of an atom = half the distance between the nuclei of two atoms of the same element that are joined together by a single covalent bond
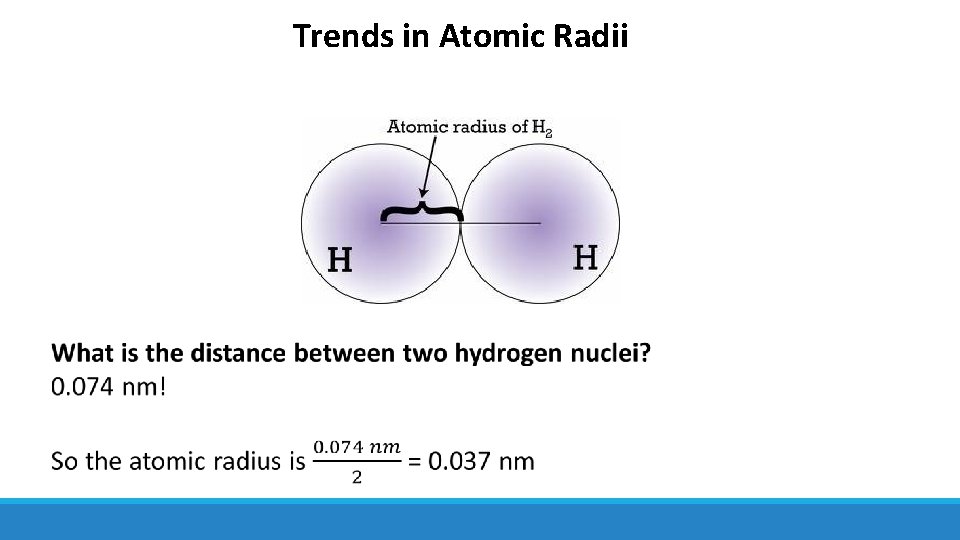
Trends in Atomic Radii
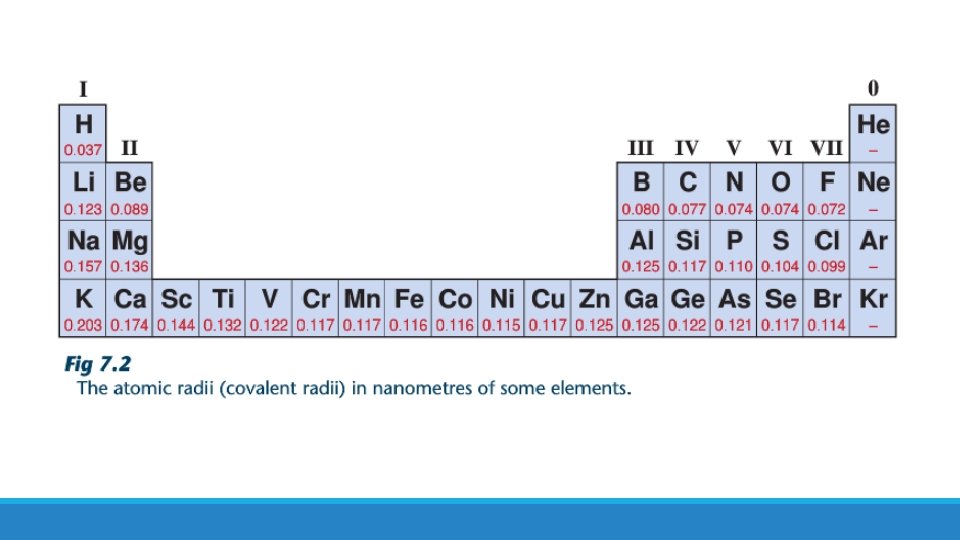
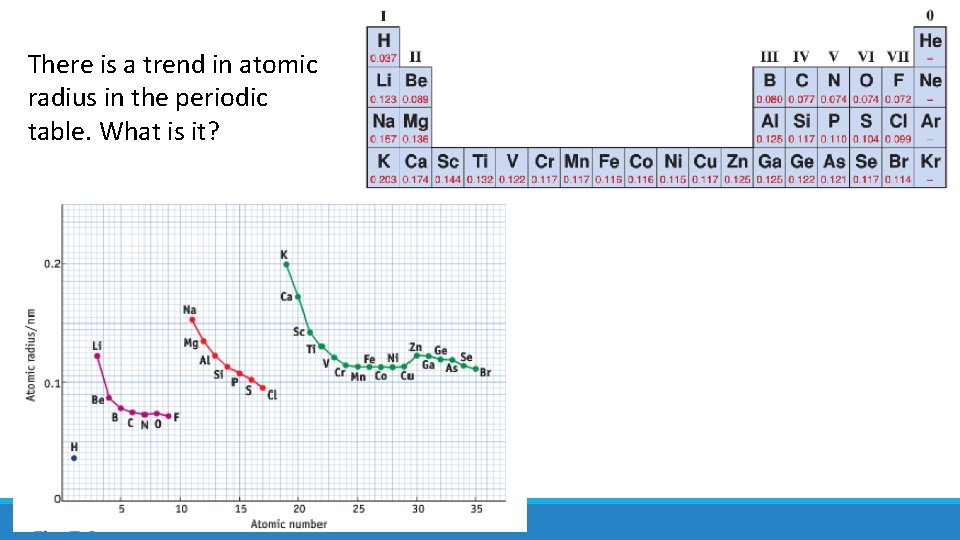
There is a trend in atomic radius in the periodic table. What is it?
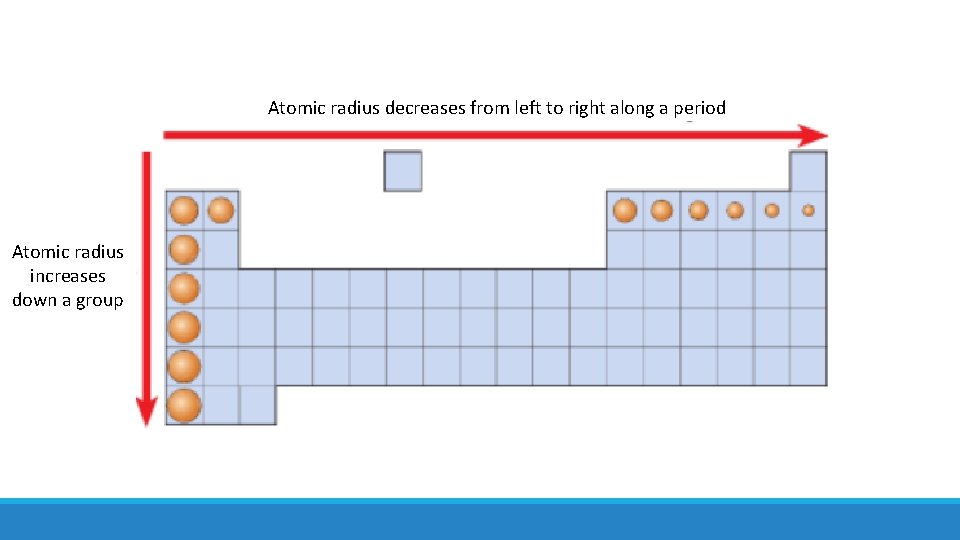
Atomic radius decreases from left to right along a period Atomic radius increases down a group
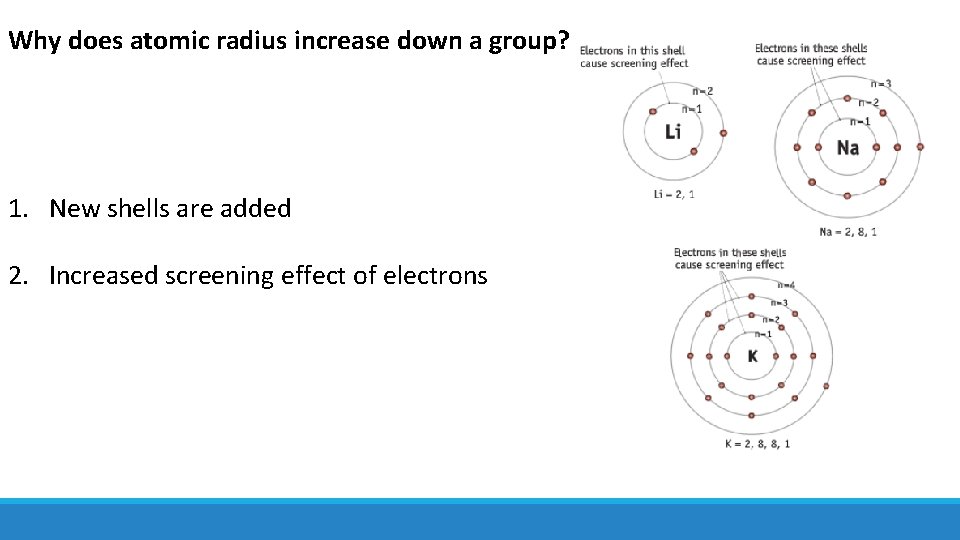
Why does atomic radius increase down a group? 1. New shells are added 2. Increased screening effect of electrons
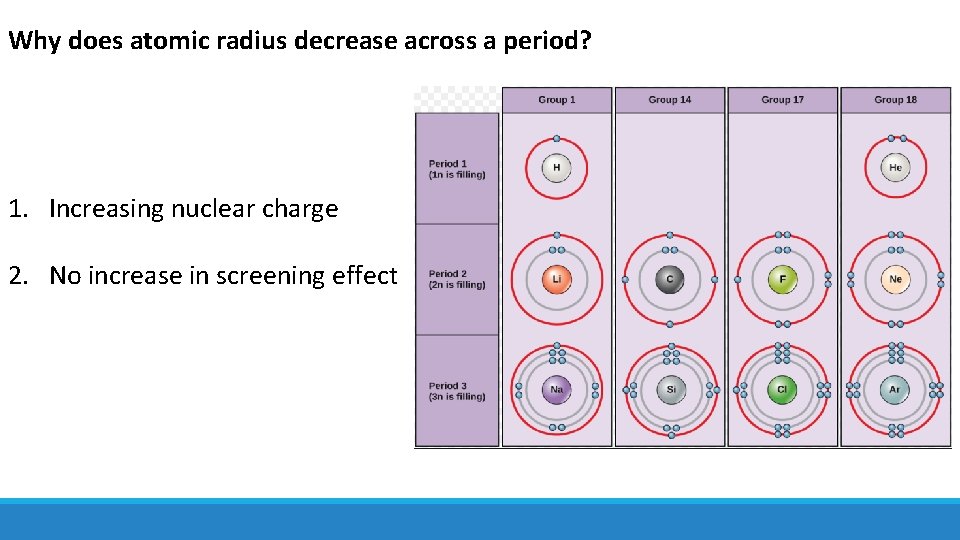
Why does atomic radius decrease across a period? 1. Increasing nuclear charge 2. No increase in screening effect
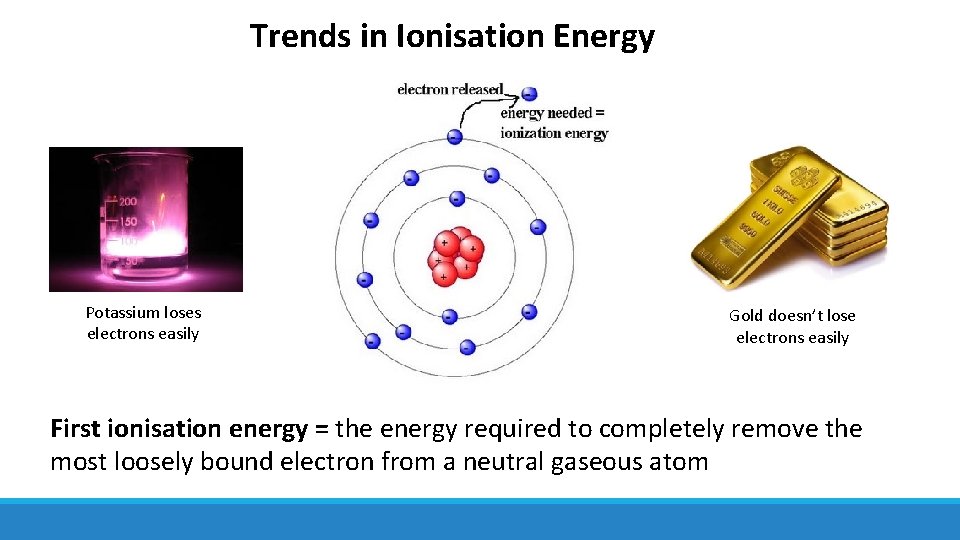
Trends in Ionisation Energy Potassium loses electrons easily Gold doesn’t lose electrons easily First ionisation energy = the energy required to completely remove the most loosely bound electron from a neutral gaseous atom
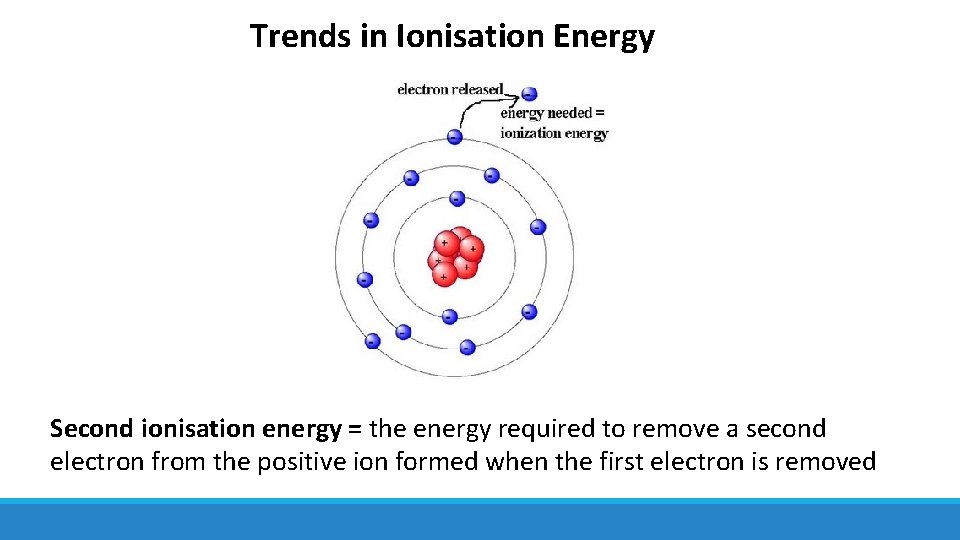
Trends in Ionisation Energy Second ionisation energy = the energy required to remove a second electron from the positive ion formed when the first electron is removed
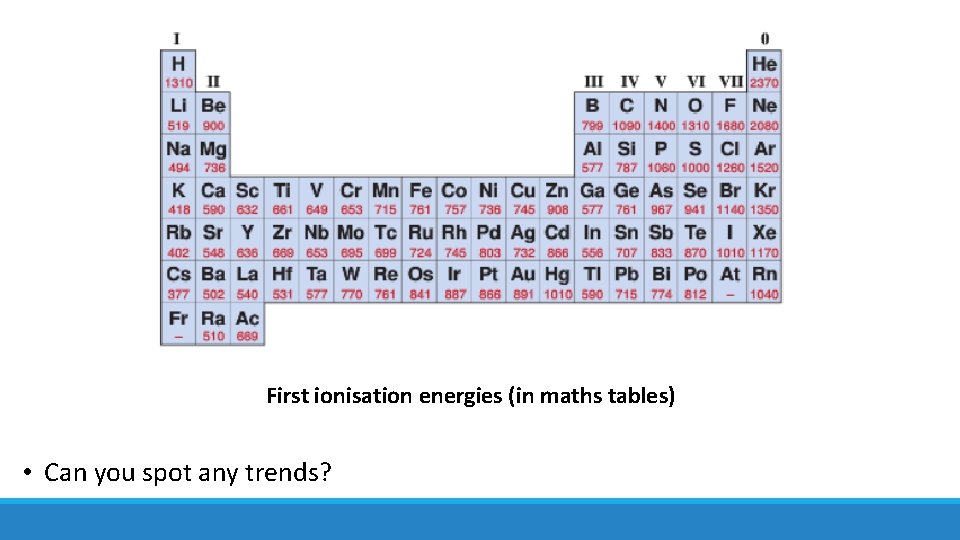
First ionisation energies (in maths tables) • Can you spot any trends?
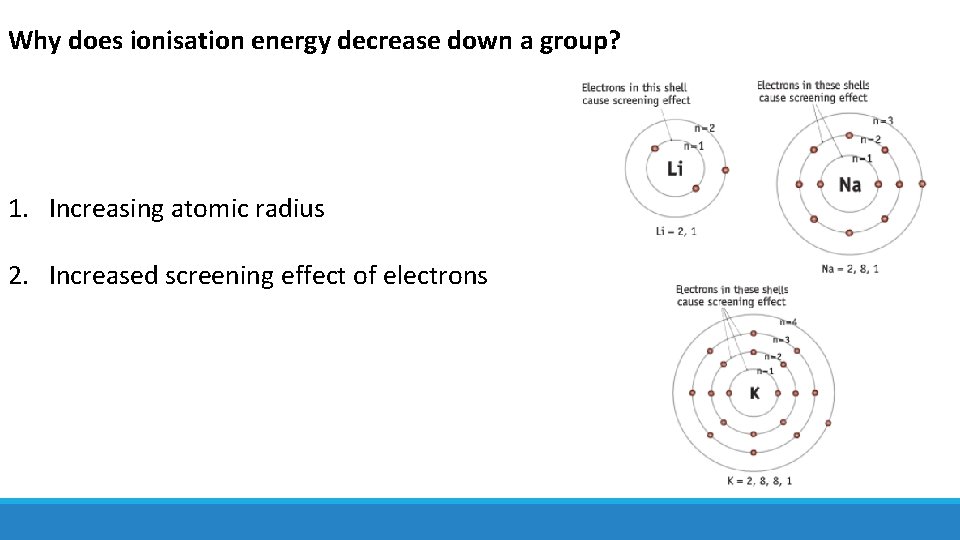
Why does ionisation energy decrease down a group? 1. Increasing atomic radius 2. Increased screening effect of electrons
Why does ionisation energy increase across the periods? 1. Increasing nuclear charge 2. Decreasing atomic radius
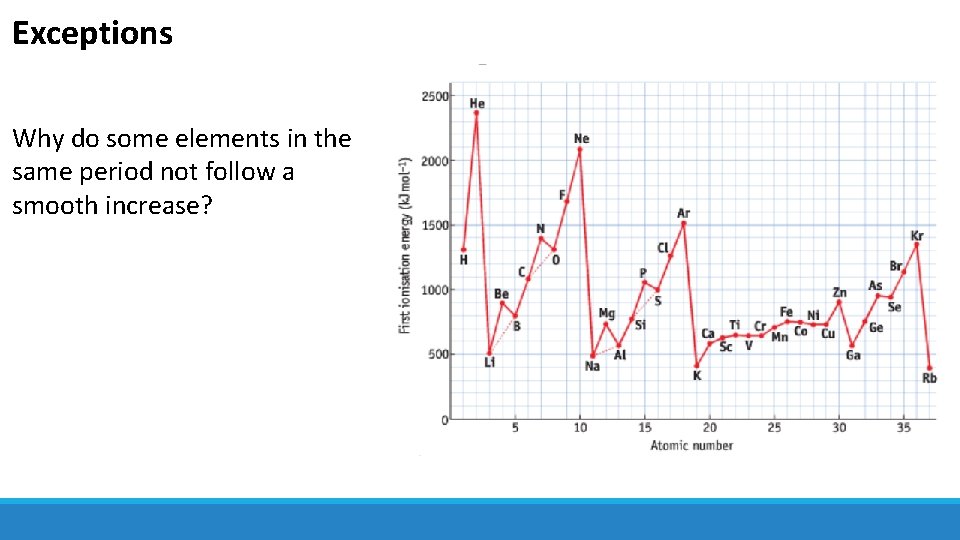
Exceptions Why do some elements in the same period not follow a smooth increase?
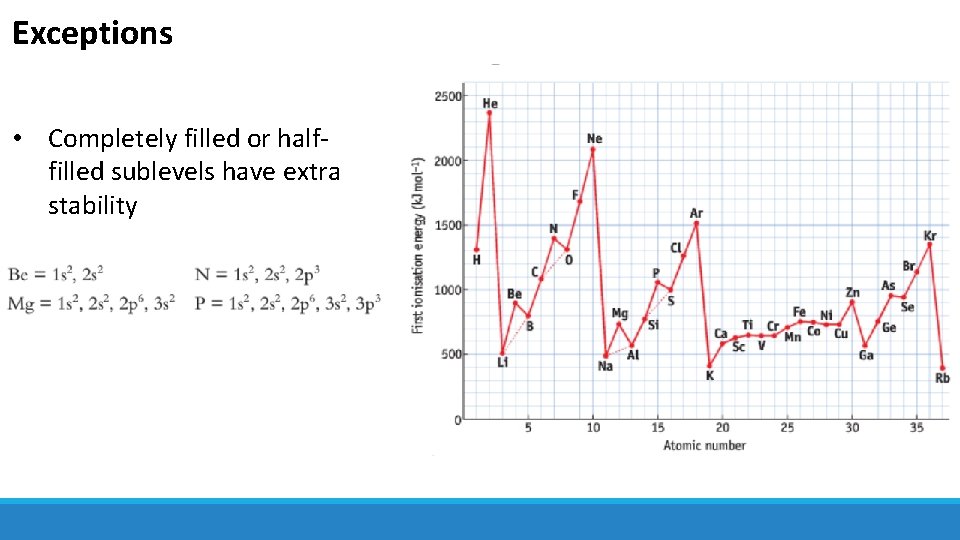
Exceptions • Completely filled or halffilled sublevels have extra stability
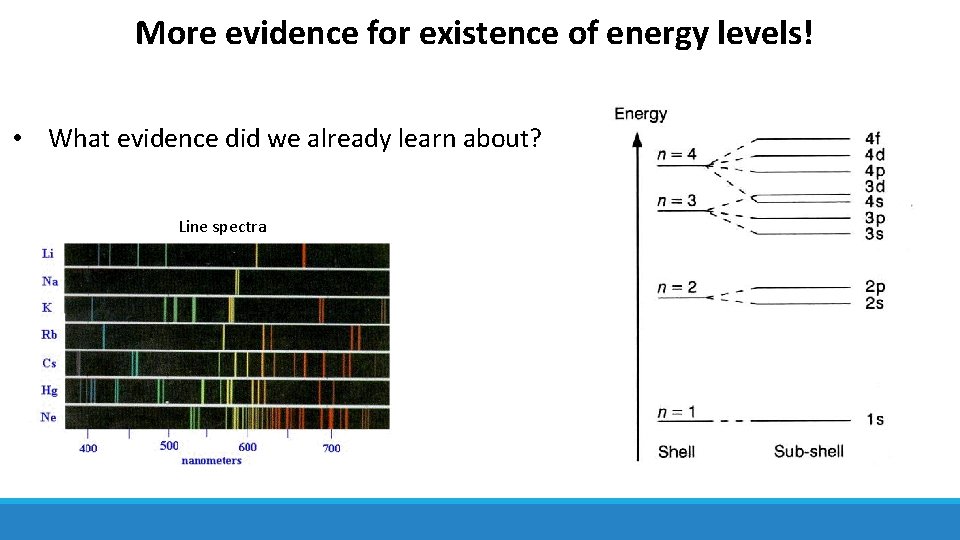
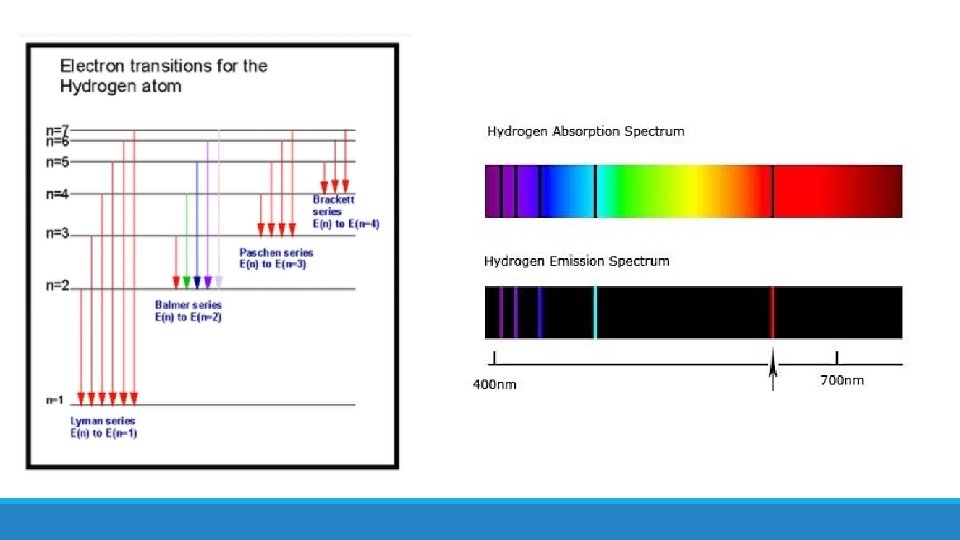
More evidence for existence of energy levels! • What evidence did we already learn about? Line spectra
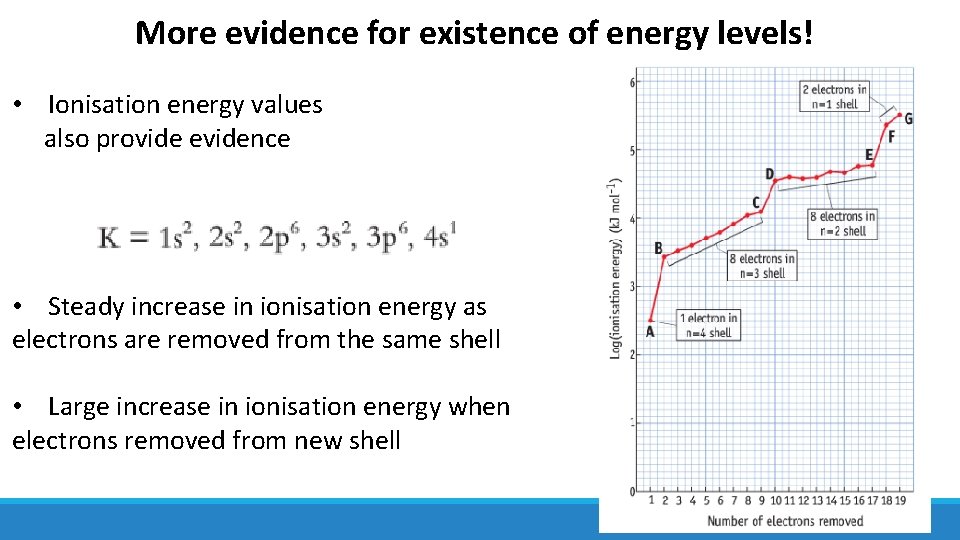
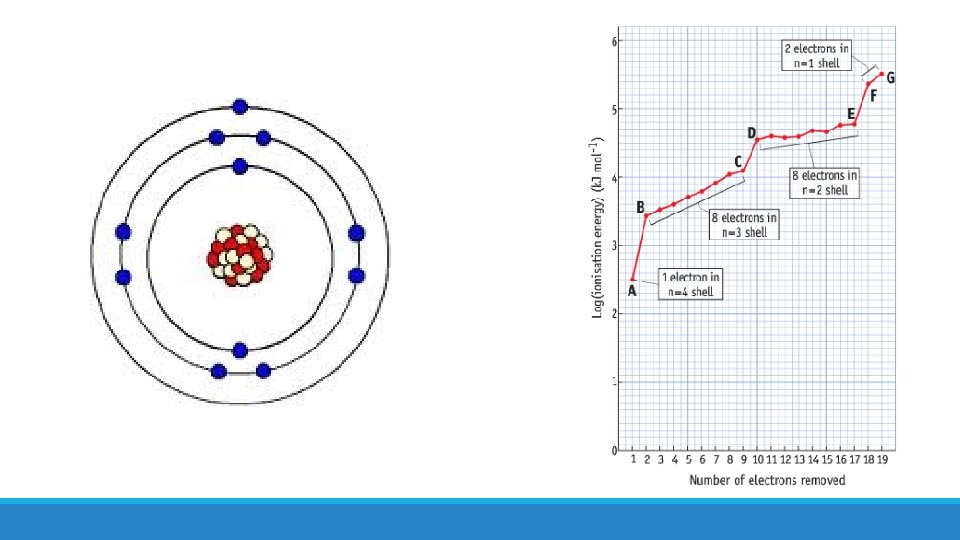
More evidence for existence of energy levels! • Ionisation energy values also provide evidence • Steady increase in ionisation energy as electrons are removed from the same shell • Large increase in ionisation energy when electrons removed from new shell
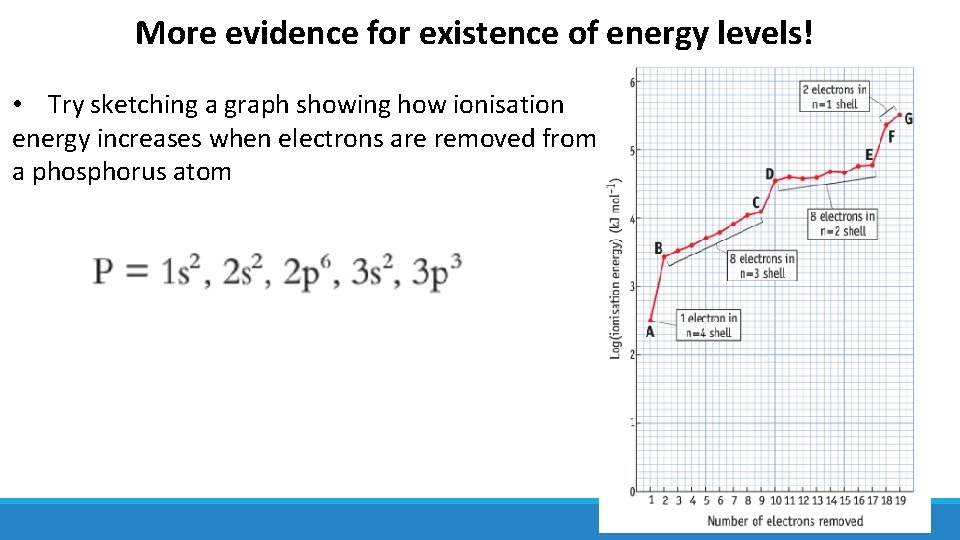
More evidence for existence of energy levels! • Try sketching a graph showing how ionisation energy increases when electrons are removed from a phosphorus atom

Now try this! Ionisation energies are on p 82 in book
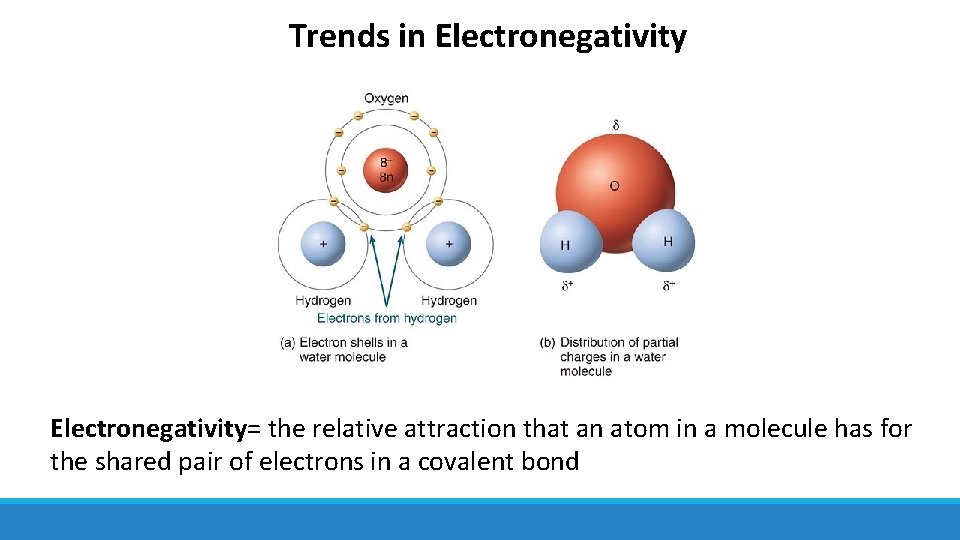
Trends in Electronegativity= the relative attraction that an atom in a molecule has for the shared pair of electrons in a covalent bond
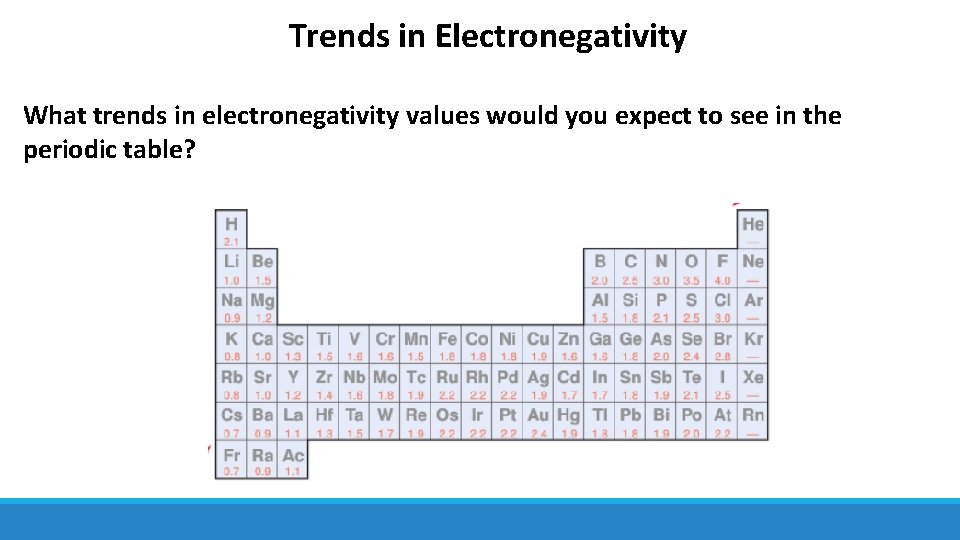
Trends in Electronegativity What trends in electronegativity values would you expect to see in the periodic table?
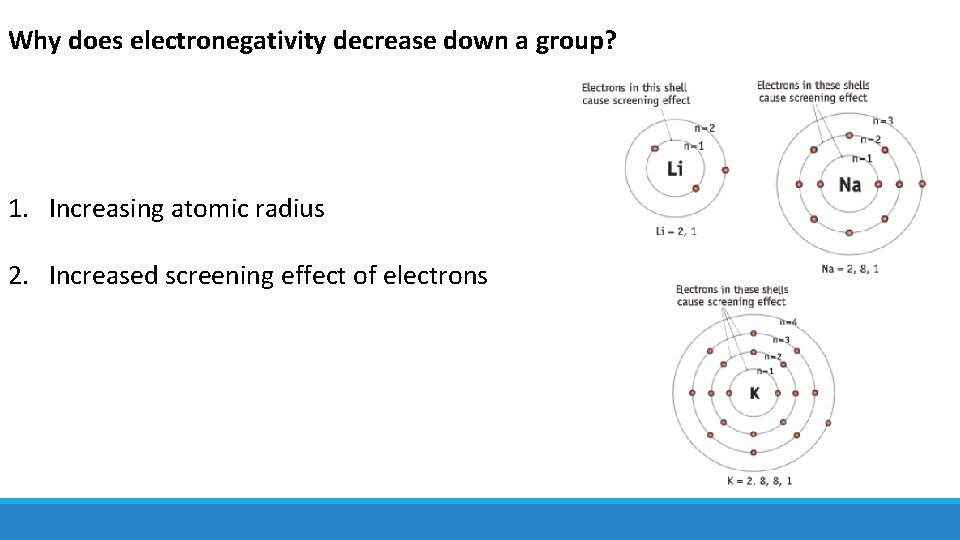
Why does electronegativity decrease down a group? 1. Increasing atomic radius 2. Increased screening effect of electrons
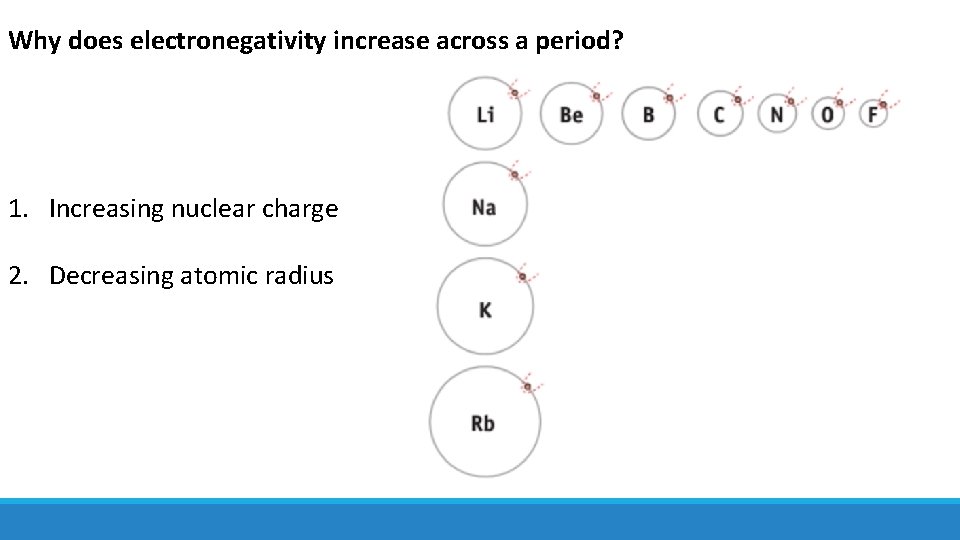
Why does electronegativity increase across a period? 1. Increasing nuclear charge 2. Decreasing atomic radius
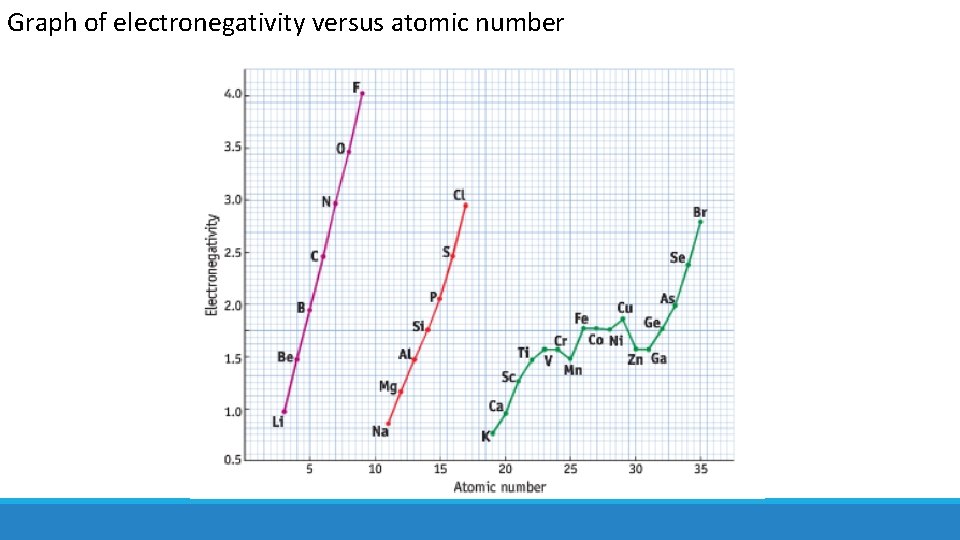
Graph of electronegativity versus atomic number

Trends within groups • What determines the chemical properties of an element? • The no. of electrons in the outer shell We will now examine the properties of: 1. Alkali metals (1 electron in outer shell) 2. Halogens (7 electrons in outer shell)
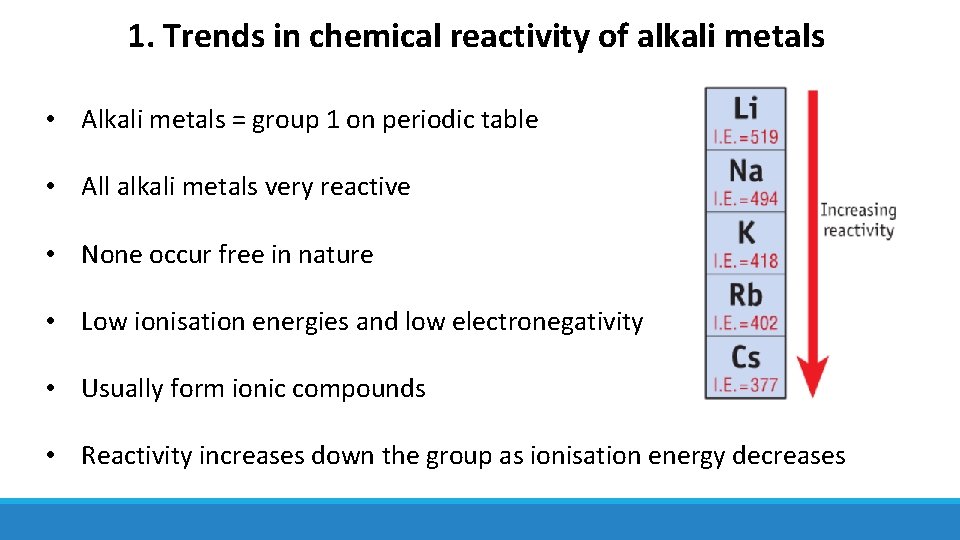
1. Trends in chemical reactivity of alkali metals • Alkali metals = group 1 on periodic table • All alkali metals very reactive • None occur free in nature • Low ionisation energies and low electronegativity • Usually form ionic compounds • Reactivity increases down the group as ionisation energy decreases
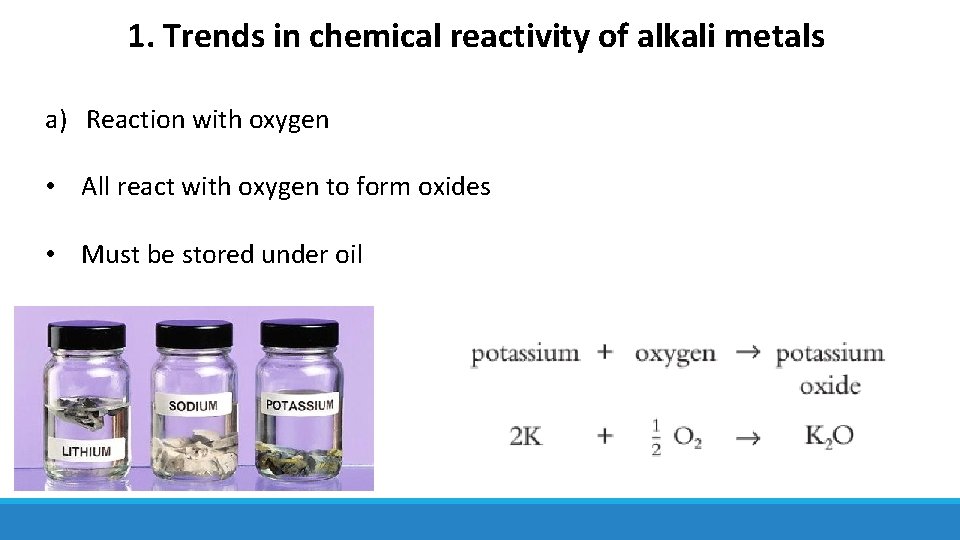
1. Trends in chemical reactivity of alkali metals a) Reaction with oxygen • All react with oxygen to form oxides • Must be stored under oil

1. Trends in chemical reactivity of alkali metals a) Reaction with water • All react with water to form the hydroxide of the metal and hydrogen gas • Reactivity increases down the group • Decreasing ionisation energy down group means electron lost more easily (ie. more reactive)

Reaction of alkali metals with acid: http: //youtu. be/X_v. Mr-3 P 0 KQ
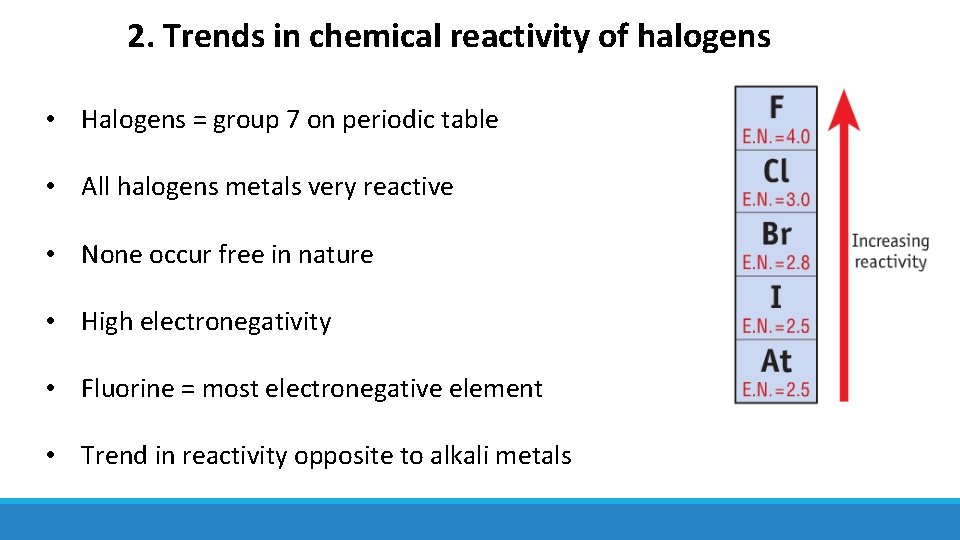
2. Trends in chemical reactivity of halogens • Halogens = group 7 on periodic table • All halogens metals very reactive • None occur free in nature • High electronegativity • Fluorine = most electronegative element • Trend in reactivity opposite to alkali metals
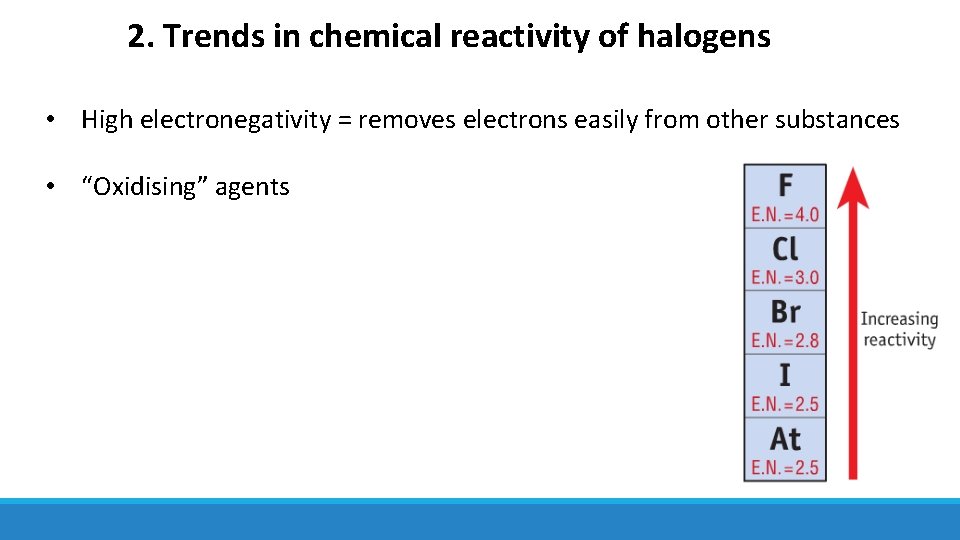
2. Trends in chemical reactivity of halogens • High electronegativity = removes electrons easily from other substances • “Oxidising” agents
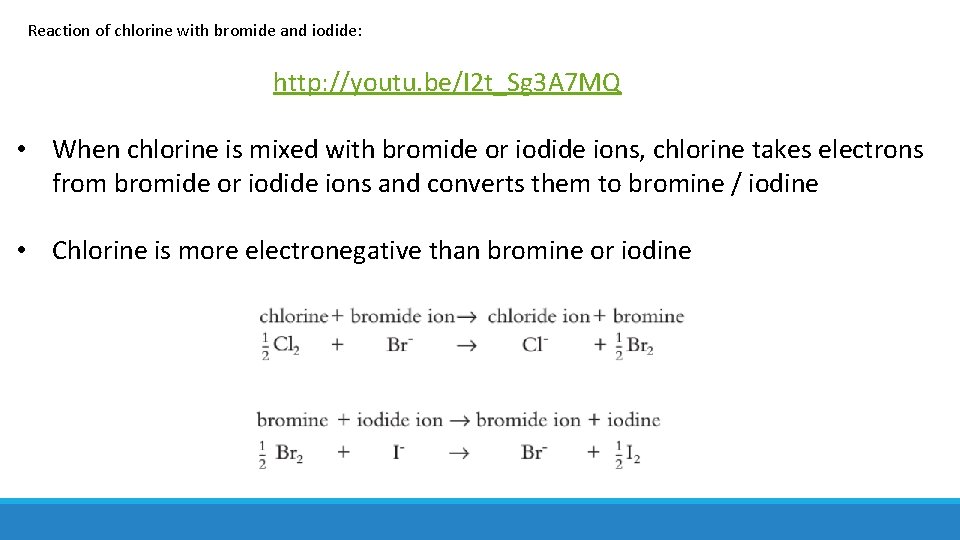
Reaction of chlorine with bromide and iodide: http: //youtu. be/I 2 t_Sg 3 A 7 MQ • When chlorine is mixed with bromide or iodide ions, chlorine takes electrons from bromide or iodide ions and converts them to bromine / iodine • Chlorine is more electronegative than bromine or iodine
3. Trends in chemical reactivity • All groups in periodic table show trends in reactivity • Example: In group 2, beryllium doesn’t react with water, magnesium reacts slowly and calcium reacts steadily with water • Reason? • ionisation energy decreases down the group
4. Trends in physical properties • Noble gases (group 8) are unreactive • Often called inert gases
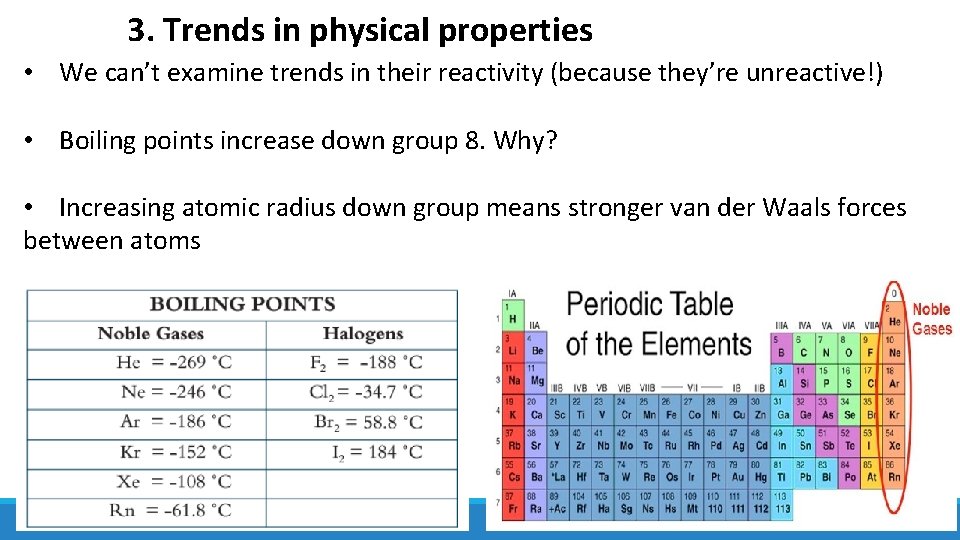
3. Trends in physical properties • We can’t examine trends in their reactivity (because they’re unreactive!) • Boiling points increase down group 8. Why? • Increasing atomic radius down group means stronger van der Waals forces between atoms
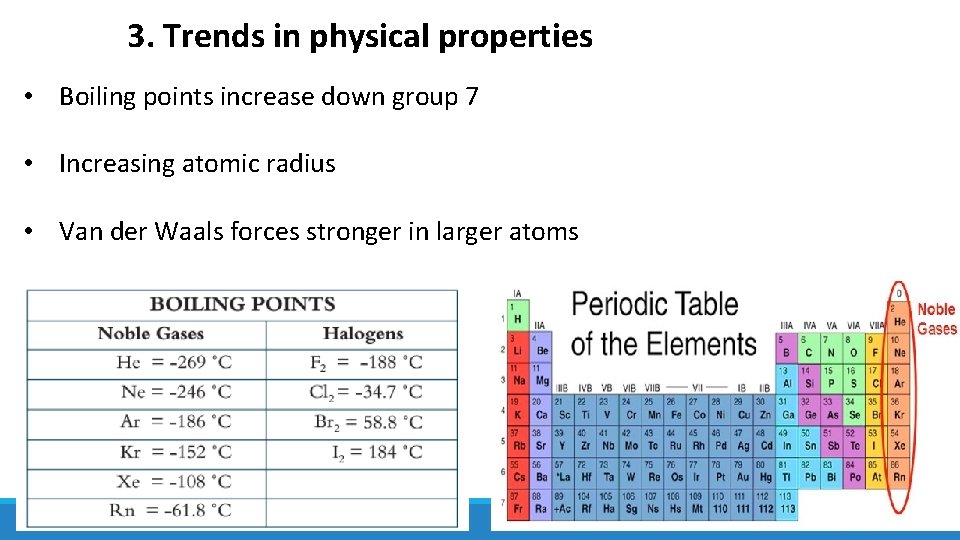
3. Trends in physical properties • Boiling points increase down group 7 • Increasing atomic radius • Van der Waals forces stronger in larger atoms
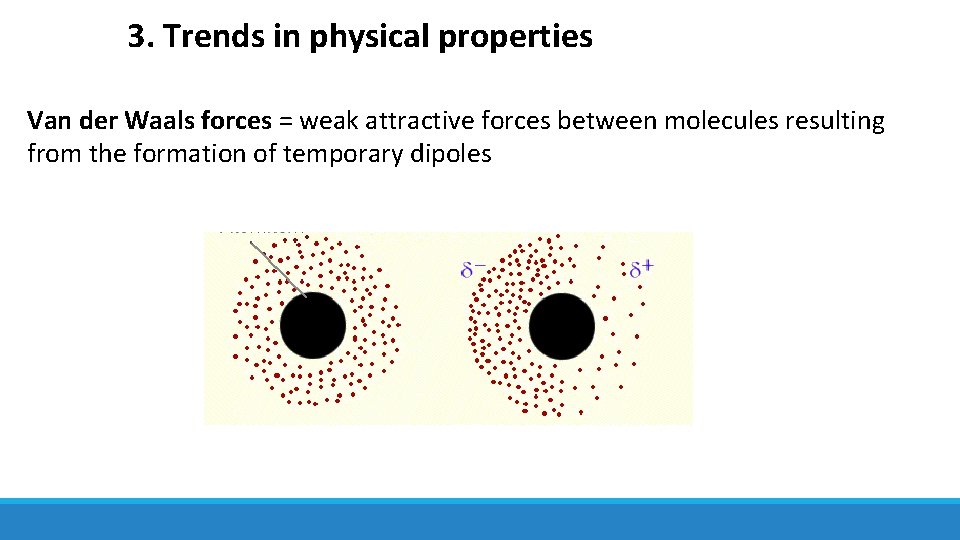
3. Trends in physical properties Van der Waals forces = weak attractive forces between molecules resulting from the formation of temporary dipoles

2005
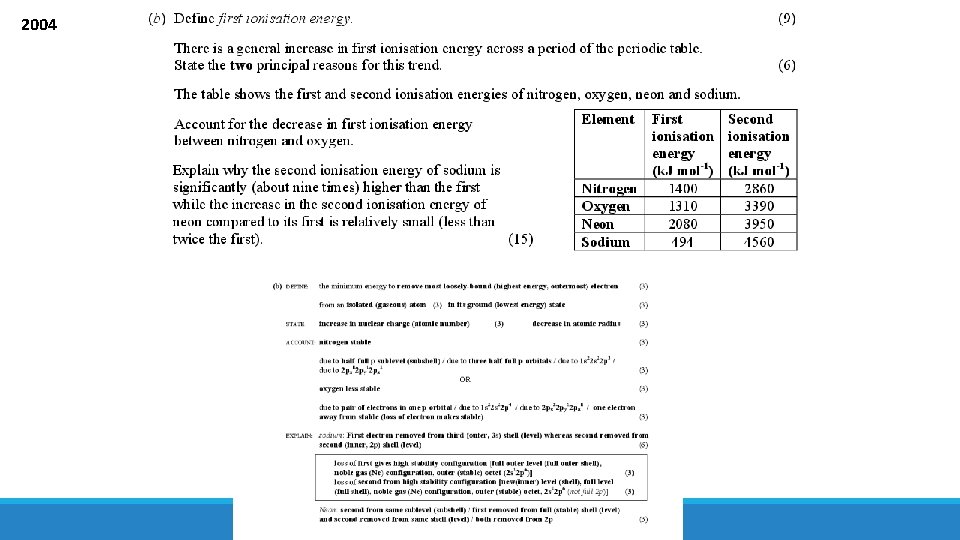
2004
- Slides: 43